More Information
Submitted: January 28, 2025 | Approved: February 06, 2025 | Published: February 07, 2025
How to cite this article: Herrera AS, Esparza MA, Arias RIS. The Inverse Relationship between Acute Myocardial Infarction and Dissolved Oxygen Levels in Water. J Nov Physiother Rehabil. 2025; 9(1): 013-023. Available from:
https://dx.doi.org/10.29328/journal.jnpr.1001066
DOI: 10.29328/journal.jnpr.1001066
Copyright license: © 2025 Herrera AS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dissociation; Heart; Hydrogen; Ischemic; Myocardial; Reperfusion; Oxygen; Water
The Inverse Relationship between Acute Myocardial Infarction and Dissolved Oxygen Levels in Water
Arturo Solís Herrera* , María del Carmen Arias Esparza and Ruth Isabel Solís Arias
, María del Carmen Arias Esparza and Ruth Isabel Solís Arias
Human Photosynthesis™ Research Center, Aguascalientes, 20000, Mexico
*Address for Correspondence: Arturo Solís Herrera, MD, PhD., Human Photosynthesis™ Research Center, Aguascalientes, 20000, Mexico, Email: [email protected]
Stroke and acute myocardial infarction are primary global causes of mortality. Statistical studies have shown that acute myocardial infarction is responsible for around 9 million deaths each year. Ischemic stroke and myocardial infarction have a significant role in global adult physical disabilities. While reperfusion is vital for tissue recovery, it may paradoxically, inadvertently increase damage through oxidative stress, inflammation, and cell death. Early reperfusion procedures are currently the sole therapy to reduce infarct size.
There are many mysteries about heart biology. It is not known the source of energy for myocardial tissues. The heart-beating force (120 mm Hg) cannot explain how erythrocytes are impelled through almost 95,000 km of capillaries in less than 5 minutes. A better knowledge of how the heart is oxygenated should allow the development of new therapies.
Myocardial Infarction (MI), colloquially known as “heart attack,” is caused by decreased or complete cessation of blood flow to a portion of the myocardium. Myocardial infarction may be “silent” and go undetected, or it could be a catastrophic event leading to hemodynamic deterioration and sudden death [1]. With coronary artery occlusion, the myocardium is deprived of oxygen. Prolonged deprivation of oxygen supply to the myocardium can lead to myocardial cell death and necrosis.
Most myocardial infarctions are due to underlying coronary artery disease, the leading cause of death in the United States. Little is known about geographic variation in Acute Myocardial Infarction (AMI) mortality within fast‐developing megacities and whether changes in healthcare accessibility correspond to changes in AMI mortality at the small‐area level [2].
Maps of the distribution of acute myocardial infarction and dissolved oxygen levels in drinking water
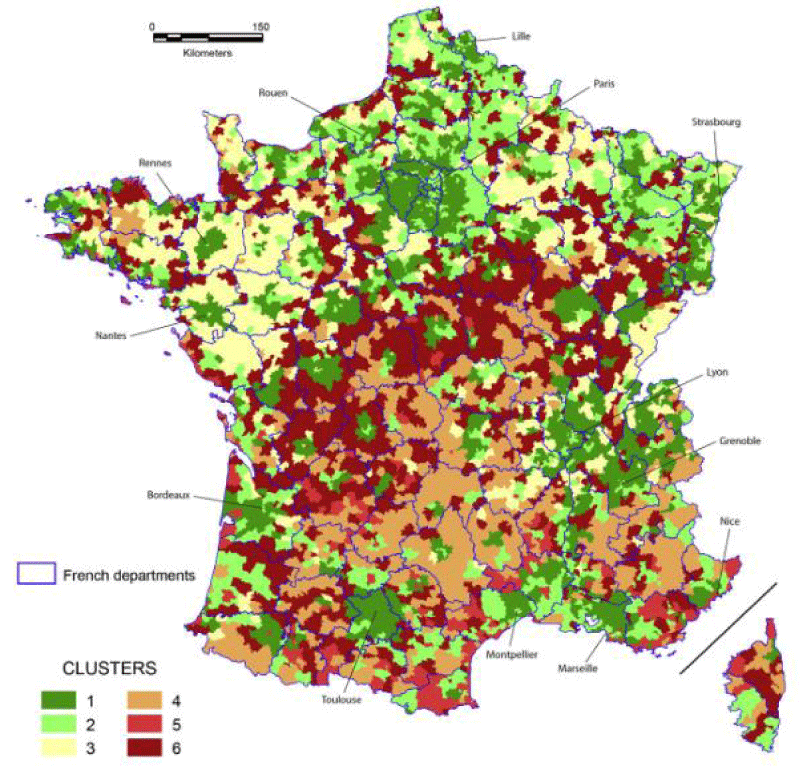
At the scale of geographic codes, the map of the standardized in-hospital prevalence of AMI in France revealed clusters with higher prevalence, notably a geographic diagonal following a northeast/southwest axis. Similarly, the spatial distribution of the standardized mortality rate shows higher mortality on a northeast/southwest diagonal line. Higher mortality was also found in the south of Normandy. A lower mortality rate after AMI was observed in the southeastern part of France [3] (Figure 1).
Figure 1: Geographical distribution in France of mortality from acute myocardial infarction, which shows areas of greater involvement. Source: Piccard, Roussot, Cottenet, et al. 2020 [3].
In France, approximately 33% of the population is supplied by large Drinking Water Treatment Plants (DWTPs) fed by surface water, and 67% by smaller DWTPs fed by groundwater [4]. Because of its higher organic matter, surface water has a greater potential for unwanted and potentially toxic Chlorination By-Product (CBP) formation than groundwater and therefore lowers the dissolved oxygen levels. Some karstic and alluvial groundwater bodies influenced by surface water may also contain a non-negligible amount of organic matter. The CBP formation potential depends mainly on the effectiveness of the treatment process used to eliminate organic matter, as well as on the chlorination process. Pre-chlorination of surface water—banned in France since 2000—required higher doses of chorine than chlorination at the outlet of the DWTP and was therefore responsible for the development of many more CBPs [5].
Almost anything that falls into the water decreases its dissolved oxygen levels. So, if the water contains nitrites at high levels, or CBP, PFAS, PFOS, PFOA, fertilizers, industrial waste, organic matter, etc., we can expect that the dissolved oxygen levels are below or well below the minimum recommended level of 6 mg/L.
COD
Chemical Oxygen Demand (COD) is the amount of dissolved oxygen that must be present in water to oxidize chemical organic materials, like petroleum. COD is used to gauge the short-term impact wastewater effluents will have on the oxygen levels of receiving waters [6].
COD versus BOD
Like COD, biochemical Oxygen Demand (BOD) measurement can be used to estimate the amount of pollution in a water sample. COD describes the amount of oxygen required to chemically break down pollutants, while BOD indicates the amount of oxygen required to break down organic pollutants biologically with microorganisms [7].
There is a correlation between COD and BOD, however, it must be experimentally established before using one parameter to express another. Usually, COD analysis (which is a much faster and more accurate method) is used to estimate BOD using the established correlation.
Why measure Chemical Oxygen Demand (COD)?
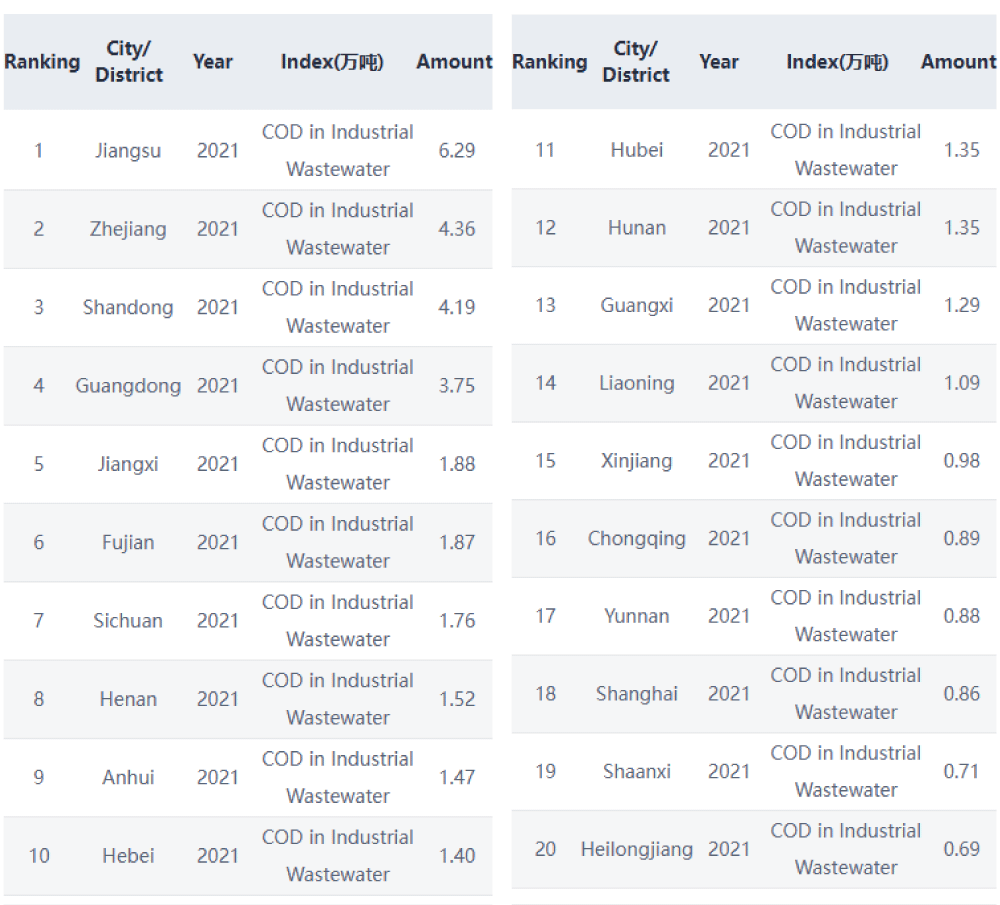
When treated wastewater is discharged into the environment, it can introduce pollution in the form of organic content to receiving waters. High levels of wastewater COD indicate concentrations of organics that can deplete dissolved oxygen in the water, leading to negative environmental and regulatory consequences. To help determine the impact and ultimately limit the amount of organic pollution in water, oxygen demand is an essential measurement (Figure 2).
Figure 2: Discharge data shows Chemical oxygen demand (COD) values in 20 cities in China. Source: https://wwwen.ipe.org.cn/mapwater/WaterCity_1.html?q=2 Retrieved: January 22, 2025.
For many people in rural China, drinking a glass of water is often a roll of the dice. Agricultural runoff and chemical waste from factories have left about 50 percent of the country’s shallow groundwater polluted, according to some estimates. Every year, tainted water makes millions of people ill around the world.
Geographical disparities in life expectancy in the USA and its relationship with DOL, CDO; and BDO
There are prominent geographic disparities in the Life Expectancy (LE) of older US adults between the states with the highest (leading states) and lowest (lagging states) LE and their causes remain poorly understood. Heart Failure (HF) has been proposed as a major contributor to these disparities [8].
Significant geographic disparities in Life Expectancy (LE) among older adults are present in the United States (US) with the highest 2017 LE observed in Hawaii and the lowest in Mississippi [9].
To characterize the geographic disparities, the leading (i.e., Hawaii, Florida, Arizona, Connecticut, Minnesota, Colorado, California, and New York) and lagging (i.e., Arkansas, Tennessee, Louisiana, Oklahoma, Kentucky, Alabama, Mississippi, and West Virginia) states were selected based on the LE at age 65 [10].
The etiologies underlying these disparities are complex, and potential causes may include human biology and genetic risk, behavioral, mental health, and socio-environmental factors, as well as variations in access to health care and healthcare utilization. Nonetheless, the reasons for the disparities between the states with the highest (leading states) and lowest (lagging states) LE are not fully understood. Heart Failure (HF) accounted for approximately one in eight deaths in the U.S. in 2017 [11] (Figure 3).
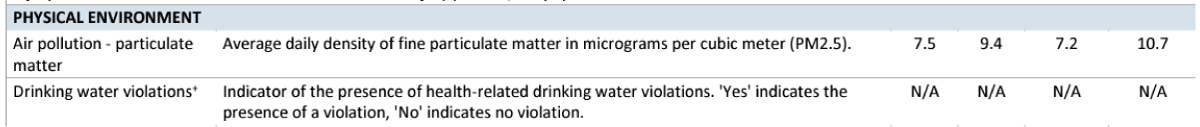
Figure 3: The quality of drinking water and therefore the levels of dissolved oxygen present in it, have not been given due importance. The fragment of the table corresponds to the state of Louisiana [12]. Usually, when the physical and chemical parameters of water or drinking water are below the recommended values, water management organisations prefer to not report them, using the N/A excuse.
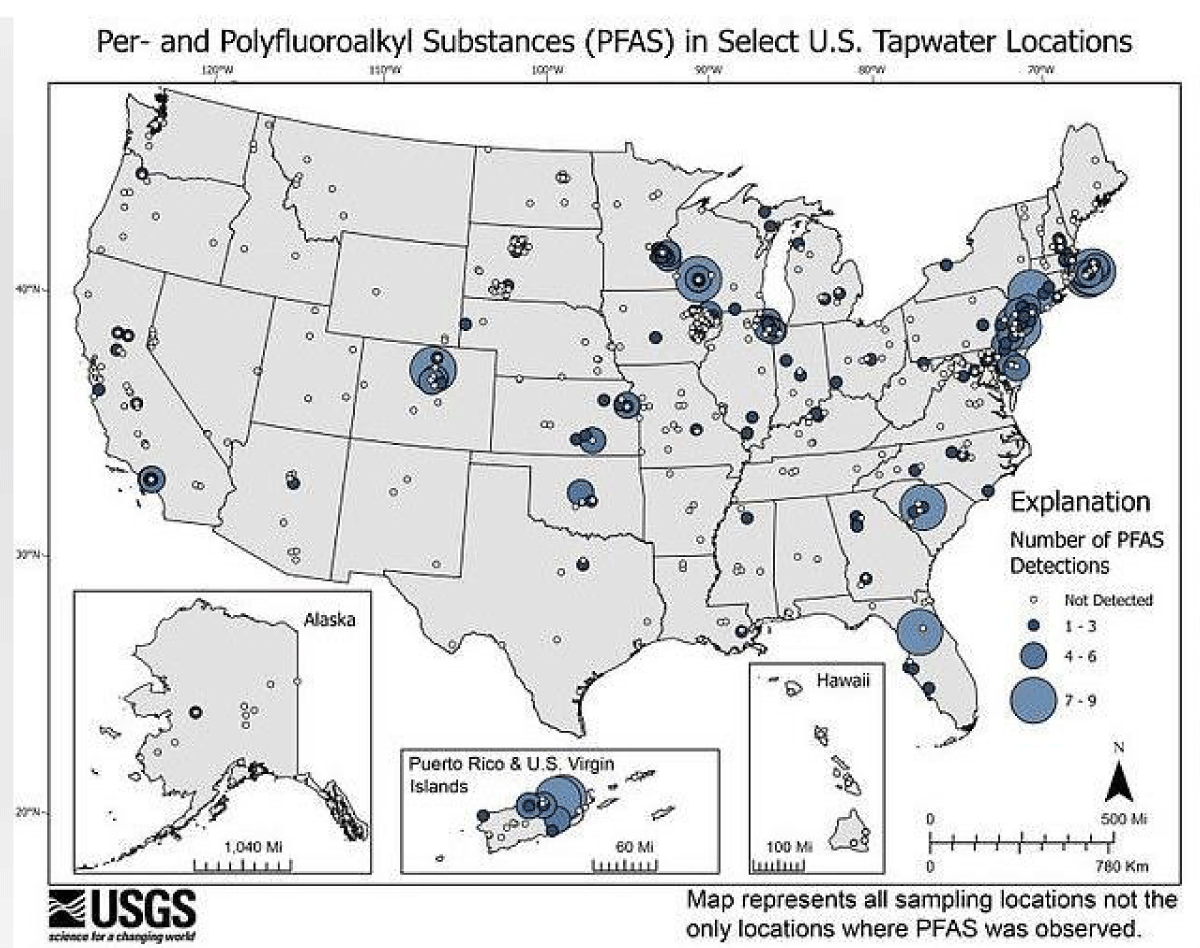
Strikingly, the states with the highest mortality from acute myocardial infarction coincide with the Stroke belt in the United States, and both with dissolved oxygen levels in drinking water well below the 6 mg/L parameter (Figures 4-6).
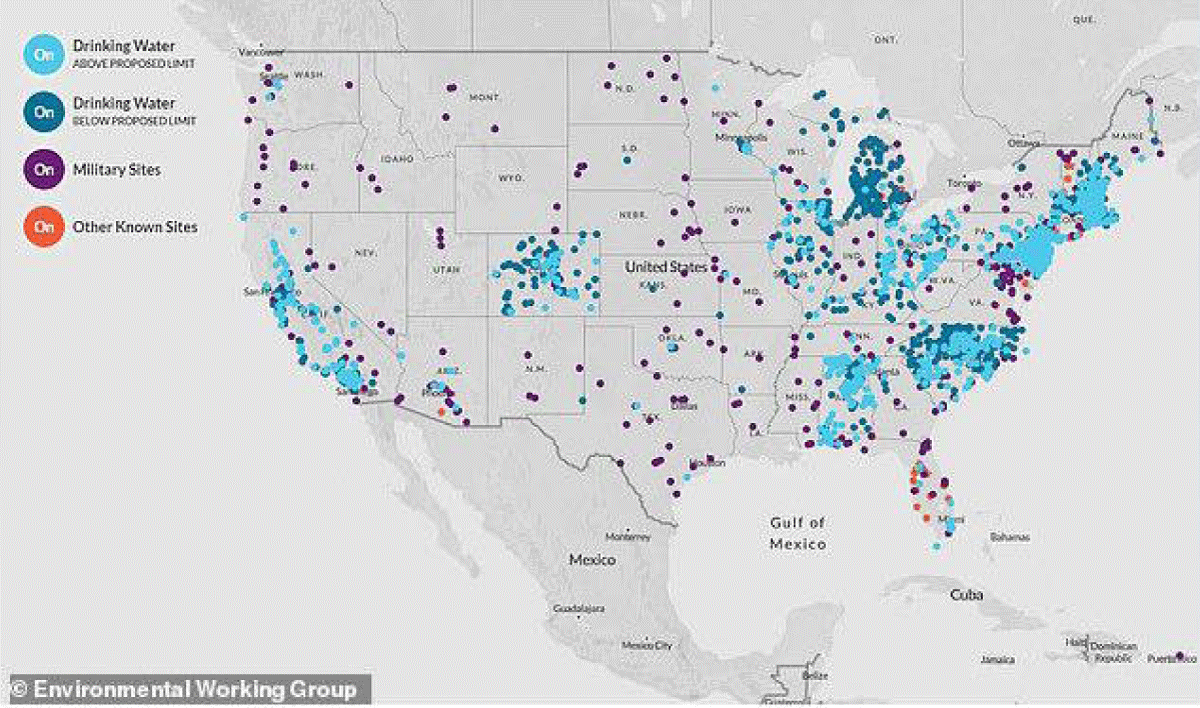
Figure 4: Map from the United States Geological Survey representing all sample locations, not only locations where PFAS was observed.
Figure 5: The contamination of drinking water, as well as its various sources (deep or superficial) by PFAS, is a constant concern in relation to human health and the environment. The distribution of PFAS levels also correlates positively with the geographic distribution of both acute myocardial infarction and stroke.
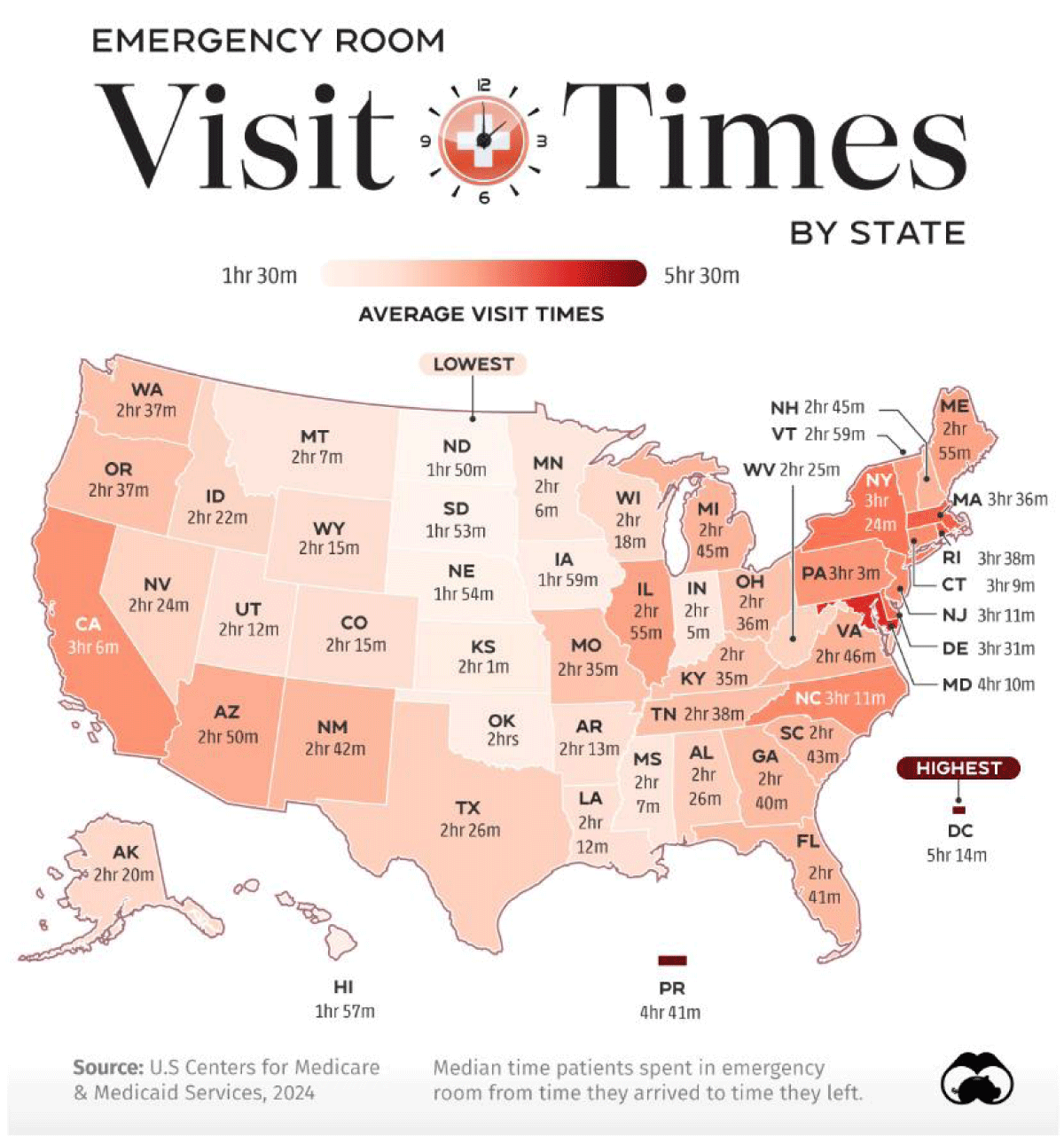
Figure 6: As expected, the geographical distribution of the frequency and time of waiting for the emergency room in the USA also correlates with low or very low levels of dissolved oxygen in water or high levels of Chemical and biological demand of oxygen. Source Visual Capitalist.
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a class of chemicals that includes more than 12,000 [13] different compounds with various chemical properties. PFAS are commonly used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they have desirable chemical properties that impart oil and water repellency, friction reduction, and temperature resistance. PFAS as a class have a wide variety of distinct chemical properties and toxicities; for example, some PFAS can bioaccumulate and persist in the human body and the environment, while others transform relatively quickly. The PFAS that do transform, however, will become one or more other PFAS because the carbon-fluorine bond they contain does not break naturally. It is for this reason that PFAS are termed “forever chemicals” [14].
Drinking water samples above the referenced PFAS levels tend to be associated with developed land and human waste indicators (artificial sweeteners and pharmaceuticals), which can be released to groundwater via septic systems. For a few samples with levels of PFOA, PFOS, and/or PFHxS > 40 ng/L, the application of wastes to agricultural land is a possible source. Thereby, human waste sources, septic systems, in particular, are important sources of perfluoroalkyl acids, especially ones with ≤8 perfluorinated carbons, in shallow groundwater [15].
There is a high correlation between the levels of PFAS in drinking water and the health level of the population (Figure 5).
Many health outcomes or conditions that were found to be associated with PFAS exposure are common in the general population. All have multiple known risk factors. The strength of the evidence for an association between PFAS and various health outcomes, therefore, all conditions with an adequately supported association should be considered for patient follow-up.
Hopefully, policymakers have become increasingly concerned regarding the widespread exposure and toxicity of per- and polyfluoroalkyl substances (PFAS) [16], given the depletive effect on the levels of dissolved oxygen in the water, and in turn increases the values of the chemical oxygen demand of the vital liquid. Decades of research have shown that environmental hazards, including exposure to toxic substances and unsafe drinking water, are disproportionately experienced according to race and ethnicity, socioeconomic status, English-language proficiency, and other forms of marginalization [17], which makes the assessment of the effects of pollutants on human health even more complex.
However, in the face of a tangle of data from which it is not possible to conclude in effective actions that improve the quality of water and therefore the health levels of the population, we can focus on raising the levels of dissolved oxygen in drinking water, since, regardless of the physical-chemical nature and name of the contaminants, invariably, the levels of dissolved oxygen in drinking water decrease, which significantly impacts the biochemical processes of the eukaryotic cell and therefore of the human body, affecting people’s well-being, even more so now that we know that our body does not take oxygen from the air that surrounds it, but from the water that our cells contain inside [18], an amazingly accurate biochemical process, which has not changed since the beginning of time because both the oxygen molecule and its generation from the water that cells contain, constitute a fundamental piece of the origin of life; but the characteristic accuracy of intracelular water dissociation is significantly altered with exposure to water with low levels of dissolved oxygen, generating an unpredictable cellular dysfunction, since alterations in oxygen levels at the intracellular level are the origin of all or almost all diseases that afflict both man and animals, including aquatic life.
Photosynthesis-like process in human beings?
It is a concept that emerged gradually, during an observational, descriptive study, about the three main causes of blindness in the world (macular degeneration, diabetic retinopathy, and glaucoma) and their possible correlation with the morphology of the tiny blood vessels that enter and leave the eye, through the optic nerve in humans. This study lasted 12 years (1990-2002) and included the ophthalmological records of almost 6000 patients (Figure 7).
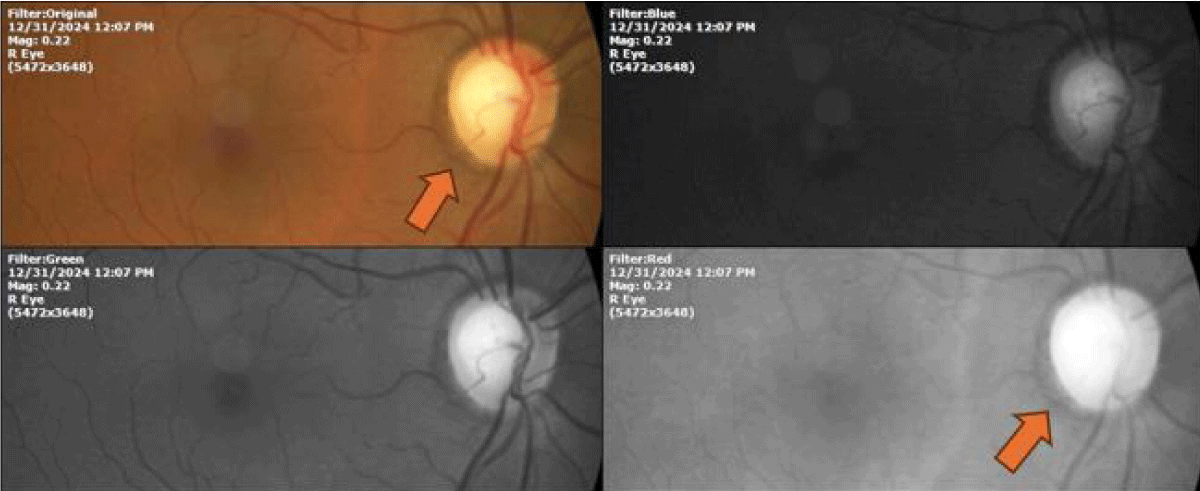
Figure 7: Fundus photograph of the right eye of a glaucomatous patient; in which a dark edge visible with the different wavelengths (orange arrow) can be seen, and which surrounds the optic nerve in its entirety. It can be said that it is melanin since it is perhaps the only known molecule that is recorded as dark in color when observed at different wavelengths, which is explained by the fact that it absorbs all the electromagnetic radiation of the visible spectrum. The name melanin comes from the Greek root melanos meaning dark.
The possibility of the unsuspected presence in prokaryotic and eukaryotic cells of molecules capable of transforming the power of light into chemical energy through the dissociation of the water molecules present inside the human cells, as in plants, It gradually emerged conformingly, thanks to the frequent observation of the fine vessels of the optic nerve, whose average diameter is 1200 microns, that is, 12 human hairs together; and the omnipresence of melanin in practically all patients, a notable detail being that these minute blood vessels respond to the presence of this pigment.
The inverse correlation between the number of vessels in the optic nerve region and the amount of melanin present in the area and vice versa finally led us to detect the surprising ability of melanin to dissociate and reassociate the water molecules present inside the cell, using visible and invisible electromagnetic radiation as an energy source, mainly from sunlight.
This reaction can be written as follows:
2H2O(liq) → 2H2(gas) + O2(gas) → 2H2O(liq) + 4e-
Melanin tolerates oxygen toxicity, which is why it dissociates and re-associates water molecules, generating 4 high-energy electrons for every two water molecules that reform [19].
But the other molecules also present inside prokaryotic and eukaryotic cells, which are capable of transforming the power of light into chemical energy that can be used by these cells, as in plants; are those compounds derived from protoporphyrin IX (PTP IX), a molecule present in all living beings, but they do not tolerate the toxicity of oxygen, expelling it into the environment; so it is said that they dissociate water molecules irreversibly [20].
This irreversible reaction can be written as follows:
2H2O(liq) → 2H2(gas) + O2(gas)
The somewhat circumstantial finding of the unsuspected ability of the human body to dissociate water molecules forces us to reconsider current biology in its entirety or at least in its majority, since the biochemistry that forms the basis of current biology and medicine, is because our body takes the oxygen it requires for its daily functioning of the atmosphere that surrounds him. But this is a crude comparison of the functioning of chimneys with the very complex human body. Thus, breathing is only to expel the CO2 that is constantly formed inside our cells.
But the much talked about gas exchange that theoretically happens in the pulmonary alveolar space [21], turns out that it is not possible or necessary, because our body takes all the oxygen it needs from the water that our cells contain inside. Thus, the amount of oxygen that each cell constantly requires to carry out its multiple and complex functions and even to replicate and maintain its shape, is equivalent, in proportion, to almost five times the amount that the atmosphere contains.
There is a constant battle of our bodies to keep up with a rising tide of pollution.
Biology of the heart
The human heart is an absolutely remarkable organ. Obviously, its main function is to pump blood throughout the body. And it does this extremely well. On average, this muscular organ will beat about 100,000 times in one day and about 35 million times in a year. During an average lifetime, the human heart will beat more than 2.5 billion times.
The circulatory system can be compared to a system of interconnected, one-way roads that range from superhighways to back alleys. Like a network of roads, the job of the circulatory system is to allow the transport of materials from one place to another. The materials carried by the circulatory system include hormones, oxygen (supposedly), cellular wastes, and nutrients from digested food. Transport of all these materials is necessary to maintain homeostasis of the body. The main components of the circulatory system are the heart, blood vessels, and blood.
Blood flows through the heart in two separate loops. The right side of the heart collects oxygen-poor blood from the body and pumps the blood to the lungs. In the lungs, carbon dioxide is released and oxygen is obtained by the blood. The left side of the heart carries the oxygen-rich blood back from the lungs and pumps it to the rest of the body. The blood delivers oxygen to the body’s cells, returning the oxygen-poor blood back to the heart.
However, the deep-seated dogma that the oxygen contained in the blood comes from the atmosphere and is taken up by the lungs does not explain the particularities of cardiac function, such as where the energy that the heart muscle requires for its prodigious function as a heart pump comes from.
Metabolism in normal heart
Under normoxic conditions, >95% of ATP generated in the heart is derived from oxidative phosphorylation in the mitochondria. The remaining 5% comes mainly from glycolysis and to a lesser extent from the citric acid cycle (Krebs cycle) [22]. The heart uses ≈60% to 70% of generated ATP to fuel contraction and the remaining 30% to 40% for various ion pumps, especially the Ca2+-ATPase in the sarcoplasmic reticulum [23]. The energy pool of the heart includes ATP (≈5 µmol/g wet weight) and phosphocreatine (PCr; ≈8 µmol/g wet weight), with the latter serving as an ATP transport and buffer system [24]. In the mitochondria, the high-energy phosphate bond in ATP can be transferred to creatine by mitochondrial creatine kinase to form PCr. With a smaller molecular weight than ATP, PCr can easily diffuse through the mitochondrial membrane into the cytosol. Here, it can be used to generate ATP from ADP through reactions catalyzed by the cytosolic creatine kinase. Because of its continuous mechanical work, the heart has a high rate of ATP hydrolysis (≈0.5 µmol/g wet weight per second). Accordingly, the high-energy phosphate pool in the heart is relatively small and can be exhausted within a few seconds. Therefore, cardiac work depends strongly on ATP generation (theoretically), and impairments in this process can rapidly induce contractile dysfunction. Approximately 70% to 90% of cardiac ATP is produced by the oxidation of Fatty Acids (FAs). The remaining 10% to 30% comes from the oxidation of glucose and lactate, as well as small amounts of ketone bodies and certain amino acids [25]. However, it is also important to note that cardiac substrate selection in human studies has been assessed mostly in the fasted state. In the fed state, when plasma levels of glucose and insulin rise, the contribution of glucose use to cardiac ATP production increases.
Unfortunately, the detailed description above is based on the fact that our body takes oxygen from the atmosphere that surrounds it, so it is 95% controversial since it is estimated that if ATP were the source of energy, the functioning of the heart alone would require approximately 6 kilograms of ATP, every 24 hours, a contradiction that has tried to be explained through alternative metabolic pathways (anaplerotic pathway, accessory pathways) but equally hypothetical.
Metabolic intermediates may act as regulators of many pathways not directly related to ATP production. Although much attention so far has focused on substrate oxidation and ATP production in the heart, it is equally important to consider that cardiac metabolism also involves anabolic processes that are essential for maintaining cellular activities and promoting cell growth and proliferation [26]. The development of Heart Failure (HF) is characterized by profound changes in cardiac structure and function. Given the fundamental role of metabolism in all of these processes, it is reasonable to assume that cardiac metabolism occupies a central position in the pathophysiology of HF. Yet it remains unclear if and how metabolic/energetic dysfunction that occurs during heart failure affects the mechanical function of the heart [27].
In large mammals, under resting conditions, the Left Ventricle (LV) consumes ATP at a rate of approximately 0.5 mmol sec−1 L−1 of cardiomyocyte [28]. This rate means that the total ATP content of the heart (at 5 mM - 10 mM) is hydrolyzed and resynthesized several times per minute. Measurements of mitochondrial respiratory capacity from human hearts have yielded conflicting results, including little or no differences in oxidative capacity between failing and non-failing hearts [29], increased capacity in failing hearts [30], and a marked reduction in capacity in failing hearts [31].
In advanced HF, there is consistent evidence of decreased myocardial ATP availability, but it remains unclear whether the reduction in ATP abundance contributes to contractile dysfunction. Approaches that would directly enhance myocardial ATP abundance in the failing heart are required to address this question. In a recent study, the xanthine oxidase inhibitor allopurinol was shown to acutely improve the relative and absolute concentrations of myocardial high-energy phosphates and ATP flux through creatine kinase in the failing human heart. Nevertheless, whether cardiac function changed in response to this energetic improvement was unknown [32]. Furthermore, in a retrospective study in a large cohort of patients with HF, Struthers, et al. [33] found no beneficial effects of long-term allopurinol treatment on cardiovascular mortality. Thereby, an alternative conclusion is that decreased levels of high-energy phosphates do not cause HF per se but represent an adaptation to decreased pump function.
Although metabolic and structural alterations in the failing heart have been increasingly well characterized, little is known about the mechanisms that drive the remodeling process in HF. Increased cardiac ROS levels have been implicated in HF, but the role of ROS in the pathogenesis of HF was questioned as a result of disappointing outcomes of antioxidant interventions in human studies [34].
The mechanistic connections between metabolic and mechanical pump function have not been firmly established [35]. If indeed impaired cardiac pumping in heart failure is in part a consequence of metabolic derangement, then understanding the underlying mechanisms will be crucial for understanding the natural history of the disease and for developing new therapies.
In short, we can summarize that the exact mechanisms linking metabolic changes to other pathological processes in HF are still poorly understood, but because the current biology of the heart is based on the fact that the oxygen inside our cells, tissues, organs, and systems of our body comes from the atmosphere when this is not chemically or physically possible.
The high concentrations of oxygen inside prokaryotic and eukaryotic cells, which in proportion are almost five times higher than the concentrations of oxygen contained in the atmosphere, have not been explained to date, except if we take into account that cells have molecules capable of dissociating the molecule from water, like in plants, and thus cover the demanding metabolic needs of the heart cell for oxygen and hydrogen.
A proof of concept is the following case, the surprising evolution of which dispels doubts in this regard.
Patient Name: E. E. L.
Date Of Birth: 04/January/2018 First consultation DATE: 13/February/2018 GENDER: Male.
Diagnosed with Down syndrome, and secondary heart problems. Skin Photo-type IV. At 5 weeks of age, they come to the doctor’s office. Dx. Down syndrome, and an eye infection, Chloramphenicol ophthalmic drops were prescribed l. 2 drops every 4 hours for 5 days. It improved a little. It started about two weeks later. He was checked by the cardiologist after 8 days. Dx. Down syndrome, they are waiting to make a karyotype. It has only one valve, it has blood communication from the atria with the ventricles, Probable surgery at 5 or 6 months of age.
The parents report hypotonia, weak crying, and could suck food for short periods, with excessive sweating.
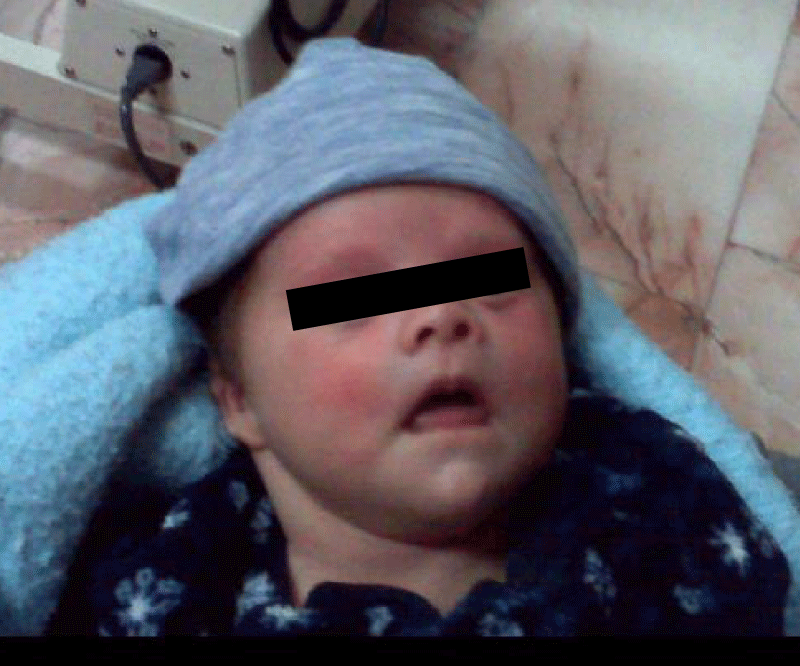
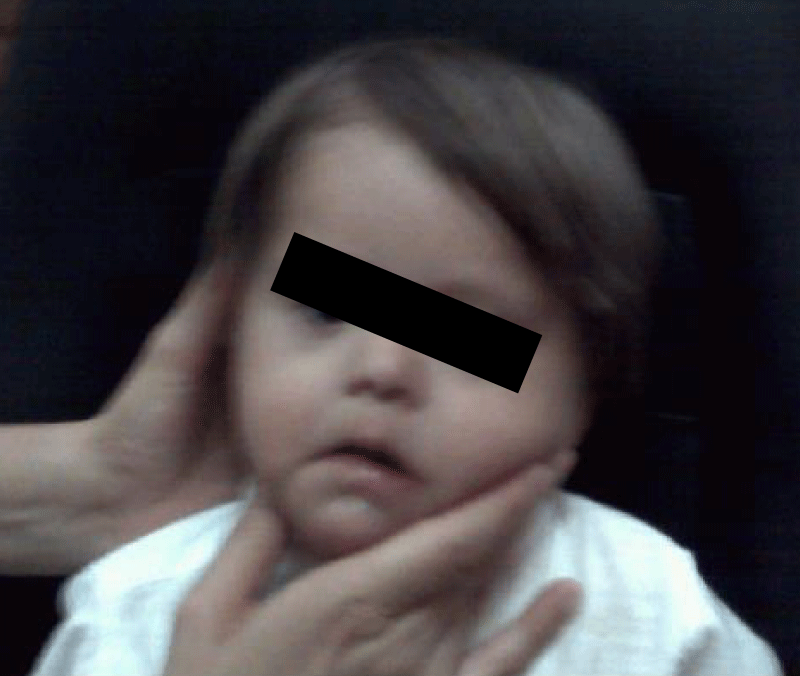
Physical examination (Figure 8) showed findings compatible with trisomy 21 (Down syndrome).
Figure 8: The patient is exhausted, with a prominent tongue.
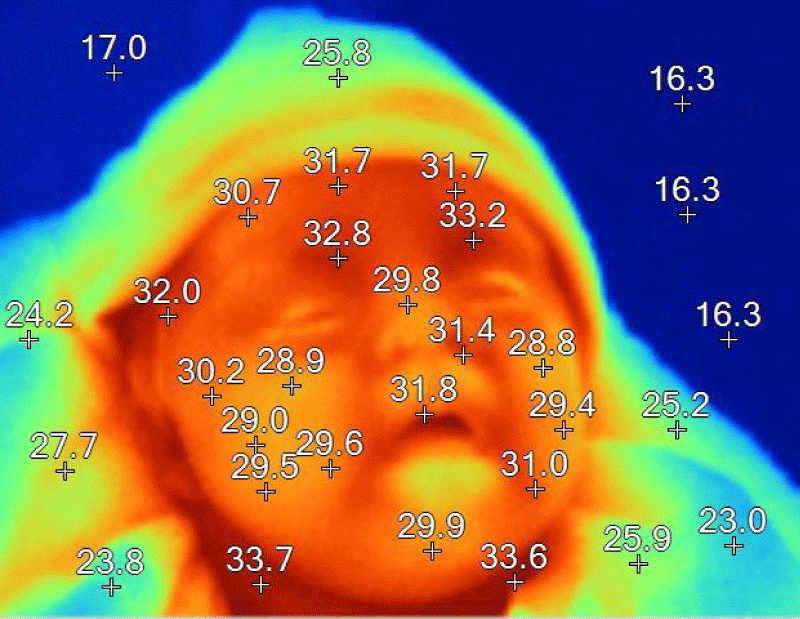
In order to obtain objective data during the physical examination, a thermography was performed, since it does not imply risk for the patient (Figure 9).
Figure 9: Temperatures are in degrees Celsius. A decrease in average temperature of between 2 and 4 degrees is observed.
The bases of our therapeutic approach, which restores oxygen (and hydrogen) levels at the intracellular level, were explained to the relatives, and once they accepted it, and signed the informed consent that the law establishes, we started the treatment, consisting of two drops orally, 6 times a day.
The parents have gone for periodic check-ups, showing a surprising improvement in the symptoms, as she began to suck the food with more force and without visible signs of exhaustion, as she was before our treatment.
The following photographs (Figures 10-15) were taken two years after the beginning of our therapeutic scheme. By then the patient was already walking and babbling.
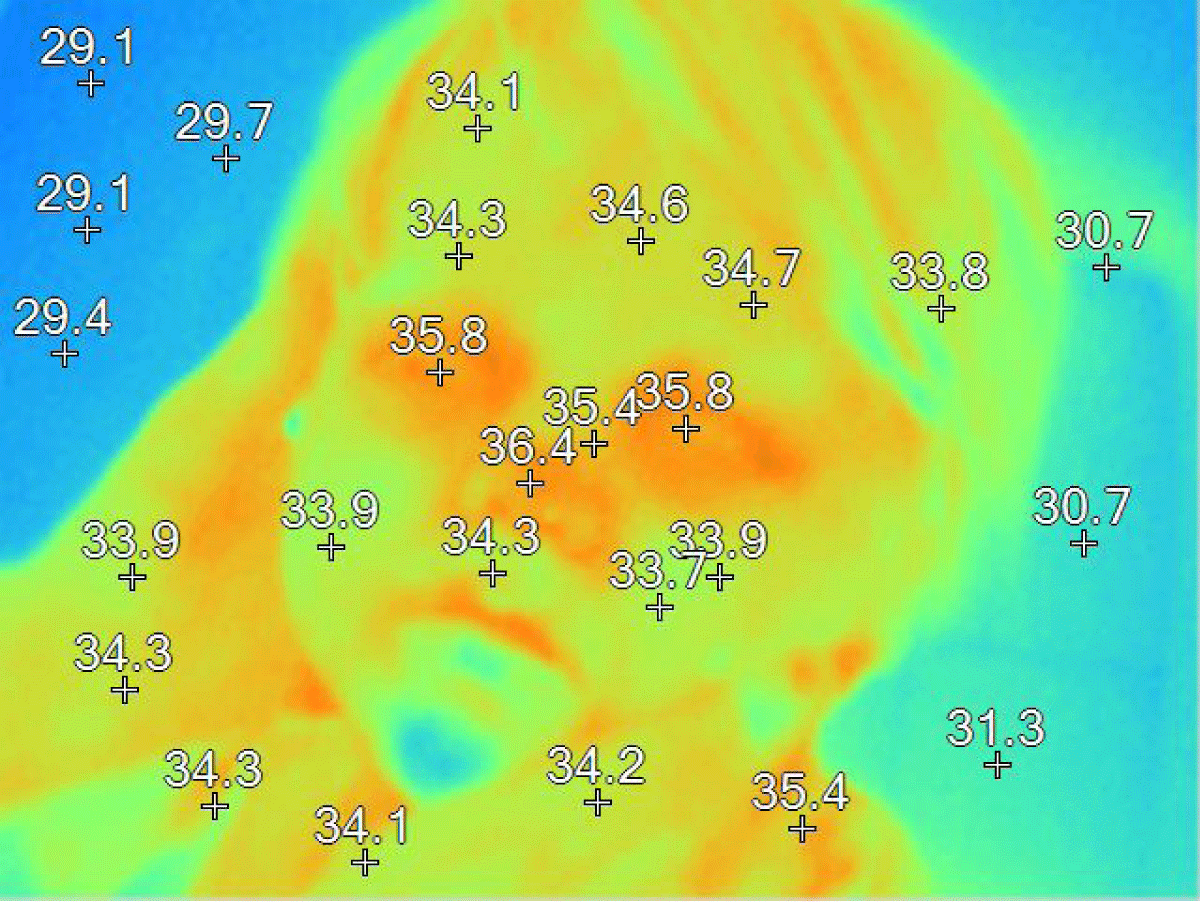
Figure 10: The thermographic image shows normal values, unlike the first thermographic image (Figure 9).
Body temperature is a reflection of the general state of the body, and therefore, the decrease in it is very significant, since hypothermia is accompanied by important functional alterations. The temperature-regulating function is very efficient, since the temperature, as well as other clinical parameters, is managed by the body within a somewhat narrow range.
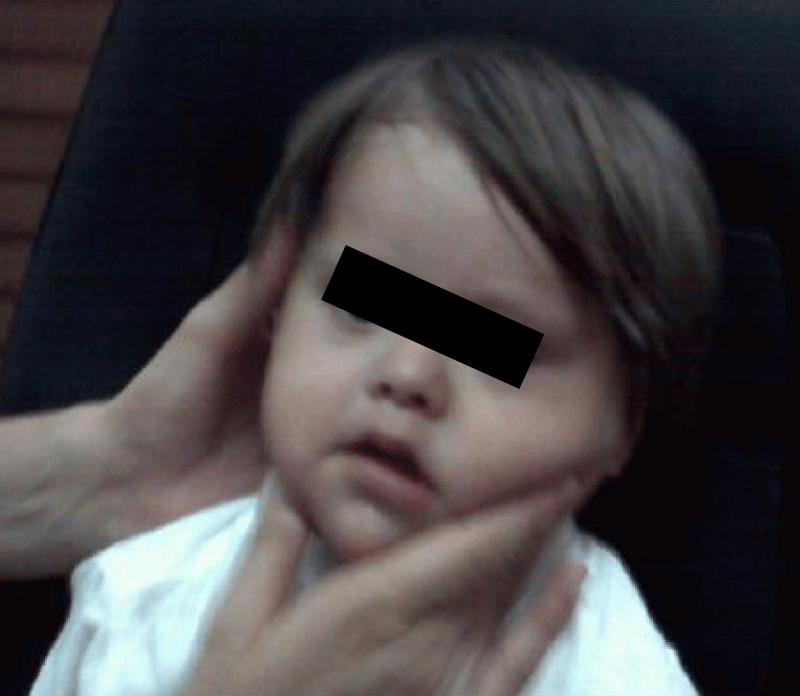
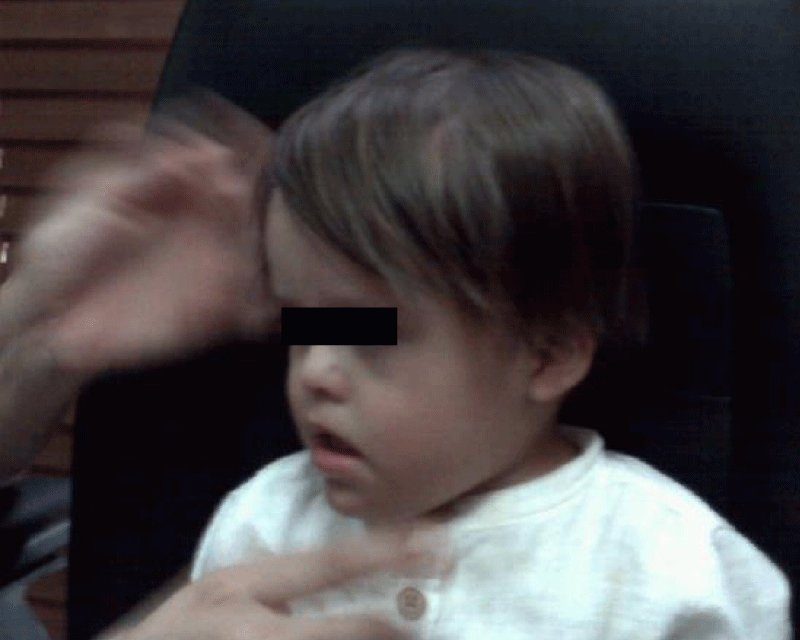
In the following Figure 11, the clinical photograph shows important changes in the usual facies of a patient diagnosed with Down Syndrome or Trisomy 21.
Figure 11: The facial expression of the patient in question is very different from that observed in patients with DOWN syndrome. For example, macroglossia has disappeared.
The measurement of body temperature by means of thermistors is an innocuous procedure that provides information on the general condition of the patient, in addition to allowing us to preserve the image of the person’s face (Figure 12).
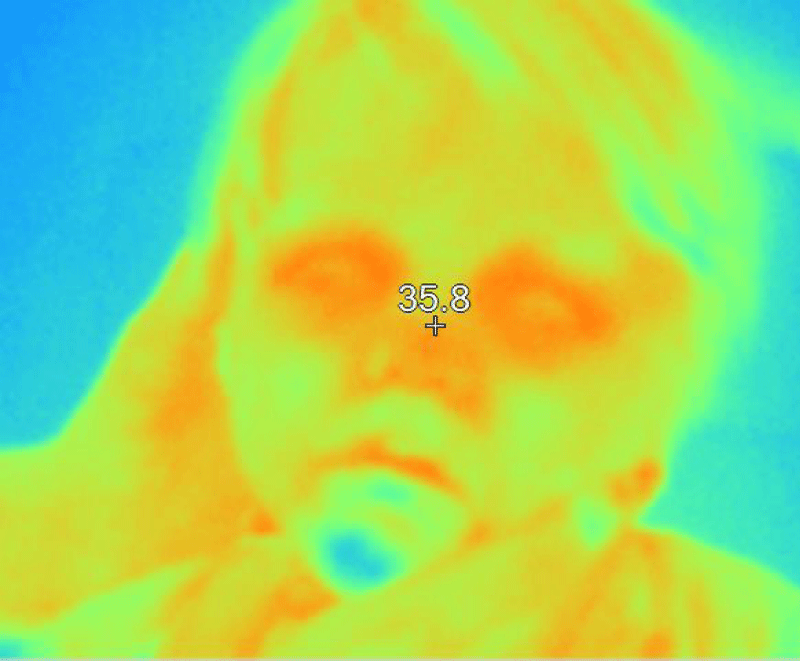
Figure 12: The thermographic image shows normal homogeneity, as well as the usual areas of higher temperature such as the eyelids, eyes, orbital area, and nasal area.
The facial expression of patients with Down syndrome has been significantly modified in the case of this patient (Figure 13).
Figure 13: The facial expression of the patient is one of the clinical characteristics that we can appreciate in patients, allowing us to assess the evolution of the health problem.
The therapeutic strategy of restoring oxygen levels at the intracellular level induces clinical changes that are noticeable to the naked eye. Clinical photography, as well as thermography, are useful procedures that allow us to objectively record the patient’s evolution, avoiding risky procedures as much as possible (Figure 14).
Figure 14: The temperature of the facial area shows normal or near-normal uniformity. The blue areas are areas of lower temperature and correspond to the hair.
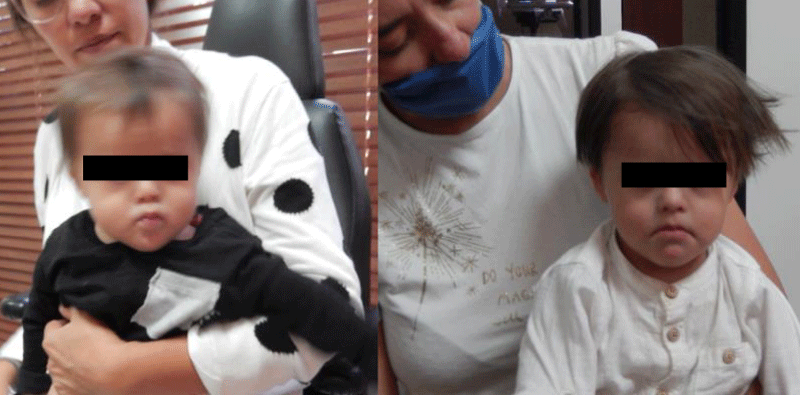
Clinical photographs also allow us to evaluate the patient’s attitude, which in this case, has evolved very favorably (Figures 15,16).
Figure 15: The patient's attitude is to some extent reflective, which is not very common in cases of Down syndrome.
Figure 16: The patient's attitude is to some extent reflective, which is not very common in cases of Down syndrome.
Comment
It is rare that, when evaluating a patient, we think about the levels of dissolved oxygen in it. Usually, the clinician focuses on reaching a diagnosis, thinking that in this way, they will be able to access a specific treatment.
But the discovery of molecules inside eukaryotic and prokaryotic cells, capable of dissociating water molecules [36], forces us to take up again the role that environmental pollution plays in the various human diseases, which despite being so varied, seem to all of them begin when oxygen levels at the intracellular level decrease mainly due to contaminated water, polluted air, and food contamination.
Water contamination with various contaminants including metals, pesticides, fertilizers, surfactants, disinfectants, phenolic substances, dyes, pharmaceuticals, hormones, endocrine disrupters, and other emerging pollutants is a major current problem affecting water quality [37]. These substances are released into the environment via diverse routes, e.g., agricultural activities, industries, hospitals, and individual households, and are found in various environmental compartments despite the decontamination treatments applied to both industrial and urban water sources [38]. Urban wastewater treatment plants are a main qualitative and quantitative point source of contaminants, notably for contaminants of emerging concern, released to surface water bodies [39]. More than ever, it is necessary to treat upstream industrial pollution as much as possible before it reaches urban treatment plants.
If we want to solve environmental problems, we must start at the village level. We aim to serve our communities and bring change for future generations, helping them understand the big environmental challenges we face, but also those solutions are within the reach of each of us. Until the discovery of the unsuspected ability of the human body to dissociate water molecules, such as plants, water pollution was not perceived as serious as it is, and even worse- it increases day by day.
Careful attention to the levels of dissolved oxygen in the drinking water supplied to the population will significantly raise the level of health of the population in general; the prompt and efficient resolution at these important levels will also make it possible to reduce the dangerously high levels of chemical and biochemical oxygen demand, and which the various types of orthodox water treatment plants have not been able to solve anywhere in the world.
The observation that the colder the climate, the higher general intelligence evolves [40], could be explained by the fact that in cold climates, oxygen tends to stay longer inside cold water, which benefits the environment, water, and human, animal, and plant health in several ways.
This work was supported by an unrestricted grant from Human Photosynthesis™ Research Center, Aguascalientes 20000, México. [email protected]
Conflict of interest
The therapeutic agents used to raise oxygen levels inside cells were developed at our research center.
- Ojha N, Dhamoon AS. Myocardial Infarction. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537076/
- Chang J, Deng Q, Hu P, Guo M, Lu F, Su Y, et al. Geographic Variation in Mortality of Acute Myocardial Infarction and Association With Health Care Accessibility in Beijing, 2007 to 2018. J Am Heart Assoc. 2023 Jun 20;12(12):e029769. Available from: https://doi.org/10.1161/jaha.123.029769
- Piccard M, Roussot A, Cottenet J, Cottin Y, Zeller M, Quantin C. Spatial distribution of in- and out-of-hospital mortality one year after acute myocardial infarction in France. Am J Prev Cardiol. 2020;2:100037. Available from: https://doi.org/10.1016/j.ajpc.2020.100037
- Ministry of Health. Drinking Water in France 2005–2006. 2008;63. [cited 20 Nov 2017]. Available from: http://solidarites-sante.gouv.fr/IMG/pdf/bilanqualite_05_06.pdf
- EFSA. 2020. [retrieved: January 27, 2025]. Available from: https://www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake
- Lacalamita D, Mongiovi C, Frini G. Front. Environ. Sci., 07 Apr 2024, Sec. Water and Wastewater Management. 2024;12. Available from: https://doi.org/10.3389/fenvs.2024.1387041
- Sibil R, Berkun M, Bekiroglu S. The comparison of different mathematical methods to determine the BOD parameters, a new developed method and impacts of these parameters variations on the design of WWTPs. Appl Math Model. 2014;38:641-658. Available from: https://doi.org/10.1016/j.apm.2013.07.013
- Yu B, Akushevich I, Yashkin AP, Yashin AI, Lyerly HK, Kravchenko J. Epidemiology of geographic disparities in heart failure among US older adults: a Medicare-based analysis. BMC Public Health. 2022;22(1):1280. Available from: https://doi.org/10.1186/s12889-022-13639-2
- World Population Review. Life expectancy by state 2020. 2020. [cited 6 Aug 2020]. Available from: https://worldpopulationreview.com/state-rankings/life-expectancy-by-state
- Yu B, Akushevich I, Yashkin A, Yashin A, Lyerly H, Kravchenko J. Heart failure mortality, incidence, prevalence, and survival in the United States: analysis of geographic disparities among older adults using Medicare data. In: Population Association of America Annual Meeting 2021. 2020. Available from: https://doi.org/10.1186/s12889-022-13639-2
- Centers for Disease Control and Prevention. Heart Failure. 2019. [cited 20 May 2020].
- University of Wisconsin, Population Health Institute. Louisiana, 2022, State Report. 2022 County Health Rankings for the 64 Ranked Parishes in Louisiana. Retrieved Jan 17, 2025. Available from: https://www.countyhealthrankings.org/sites/default/files/media/document/CHR2022_LA_0.pdf
- EPA. EPA Comptox Dashboard. [accessed Jan 23, 2025]. Available from: https://comptox.epa.gov/dashboard/chemical-lists/pfasmaster
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Population Health and Public Health Practice; Board on Environmental Studies and Toxicology; Committee on the Guidance on PFAS Testing and Health Outcomes. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington (DC): National Academies Press (US); 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK584702/
- Silver M, Phelps W, Masarik K, Burke K, Zhang C, Schwartz A, et al. Prevalence and Source Tracing of PFAS in Shallow Groundwater Used for Drinking Water in Wisconsin, USA. Environ Sci Technol. 2023;57(45):17415-17426. Available from: https://pubs.acs.org/doi/10.1021/acs.est.3c02826
- Mueller R, Salvatore D, Brown P, Cordner A. Quantifying Disparities in Per- and Polyfluoroalkyl Substances (PFAS) Levels in Drinking Water from Overburdened Communities in New Jersey, 2019-2021. Environ Health Perspect. 2024;132(4):47011. Available from: https://doi.org/10.1289/ehp12787
- Agyeman J, Schlosberg D, Craven L, Matthews C. Trends and directions in environmental justice: from inequity to everyday life, community, and just sustainabilities. Annu Rev Environ Resour. 2016;41(1):321–340. Available from: https://doi.org/10.1146/annurev-environ-110615-090052
- Solís Herrera A, Arias Esparza MdC. Oxygen from the Atmosphere Cannot Pass Through the Lung Tissues and Reach the Bloodstream. The Unexpected Capacity of Human Body to Dissociate the Water Molecule. J Pulmonol Res Rep. 2022;SRC/JPRR-133. Available from: https://www.onlinescientificresearch.com/abstract/oxygen-from-the-atmosphere-cannot-pass-through-the-lung-tissues-and-reach-the-bloodstream-the-unexpected-capacity-of-human-body-to-1804.html
- Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, et al. A Eukaryote without a Mitochondrial Organelle. Curr Biol. 2016;26(10):1274–1284. Available from: https://doi.org/10.1016/j.cub.2016.03.053
- Solís Herrera A. The Biological Pigments in Plants Physiology. Agric Sci. 2015;6(6):1262–1271. Available from: https://www.scirp.org/journal/paperinformation?paperid=60724
- West JB. Three classical papers in respiratory physiology by Christian Bohr (1855-1911) whose work is frequently cited but seldom read. Am J Physiol Lung Cell Mol Physiol. 2019;316(4):L585-L588. Available from: https://doi.org/10.1152/ajplung.00527.2018
- Ingwall JS. ATP and the Heart. Norwell, Massachusetts: Kluwer Academic Publishers. 2002. Available from: https://link.springer.com/book/10.1007/978-1-4615-1093-2
- Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. Available from: https://doi.org/10.1152/physrev.1990.70.2.247
- Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. Available from: https://doi.org/10.1016/S0735-1097(02)02160-5
- Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Craig JC. Dual carbon-labeled isotope experiments using D-[6-14C] glucose and L-[1,2,3-13C3] lactate: a new approach for investigating human myocardial metabolism during ischemia. J Am Coll Cardiol. 1985;5:1138–1146. Available from: https://doi.org/10.1016/s0735-1097(85)80016-4
- Ward PS, Thompson CB. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 2012;4:a006783. Available from: https://doi.org/10.1101/cshperspect.a006783
- Lopez R, Marzban B, Gao X, Lauinger E, Van den Bergh F, Whitesall SE, et al. Impaired Myocardial Energetics Causes Mechanical Dysfunction in Decompensated Failing Hearts. Function. 2020. Available from: https://doi.org/10.1093/function/zqaa018
- Wu F, Zhang EY, Zhang J, Bache RJ, Beard DA. Phosphate metabolite concentrations and ATP hydrolysis potential in normal and ischaemic hearts. J Physiol. 2008;586(17):4193–4208. Available from: https://doi.org/10.1113/jphysiol.2008.154732
- Holzem KM, Vinnakota KC, Ravikumar VK, Madden EJ, Ewald GA, Dikranian K, et al. Mitochondrial structure and function are not different between nonfailing donor and end-stage failing human hearts. FASEB J. 2016;30(8):2698–2707. Available from: https://doi.org/10.1096/fj.201500118r
- Cordero-Reyes AM, Gupte AA, Youker KA, Loebe M, Hsueh WA, Torre-Amione G, et al. Freshly isolated mitochondria from failing human hearts exhibit preserved respiratory function. J Mol Cell Cardiol. 2014;68:98–105. Available from: https://doi.org/10.1016/j.yjmcc.2013.12.029
- Stride N, Larsen S, Hey-Mogensen M, Sander K, Lund JT, Gustafsson F, et al. Decreased mitochondrial oxidative phosphorylation capacity in the human heart with left ventricular systolic dysfunction. Eur J Heart Fail. 2013;15(2):150–157. Available from: https://doi.org/10.1093/eurjhf/hfs172
- Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. J Am Coll Cardiol. 2012;59:802–808. Available from: https://doi.org/10.1016/j.jacc.2011.10.895
- Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 2002;87:229–234. Available from: https://doi.org/10.1136/heart.87.3.229
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. Available from: https://doi.org/10.1056/nejm200001203420302
- Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113(6):709–724. Available from: https://doi.org/10.1161/circresaha.113.300376
- Herrera AS, del Carmen Arias Esparza M, Solís Arias PE, Ávila-Rodriguez M, Barreto GE, Li Y, Bachurin SO, et al. Unsuspected Intrinsic Property of Melanin to Dissociate Water Can Be Used for the Treatment of CNS Diseases. CNS Neurol Disord Drug Targets. 2016;15(2):135-140. Available from: https://doi.org/10.2174/1871527315666160202122943
- Warren-Vega WM, Campos-Rodríguez A, Zárate-Guzmán AI, Romero-Cano LA. A current review of water pollutants in the American continent: trends and perspectives in detection, health risks, and treatment technologies. Int J Environ Res Public Health. 2023;20(5):4499. Available from: https://doi.org/10.3390/ijerpH20054499
- Abily M, Acuña V, Corominas L, Rodríguez-Roda I, Gernjak W. Strategic routes for wastewater treatment plant upgrades to reduce micropollutants in European surface water bodies. J Clean Prod. 2023;415:137867. Available from: https://doi.org/10.1016/j.jclepro.2023.137867
- Rizzo L, Malato S, Antakyali D, Beretsou VG, Đolić MB, Gernjak W, et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci Total Environ. 2019;655:986–1008. Available from: https://doi.org/10.1016/j.scitotenv.2018.11.265
- Kanazawa S. Temperature and evolutionary novelty as forces behind the evolution of general intelligence. Intelligence. 2008;36(2):99–108. Available from: https://www.sciencedirect.com/science/article/pii/S0160289607000669