More Information
Submitted: January 15, 2025 | Approved: January 30, 2025 | Published: January 31, 2025
How to cite this article: Herrera AS, Esparza MA, Shuckov S. Insights into the Complexity of Paradoxical Antioxidants Behavior. And the Reasons for it’s almost Zero or no Effect on Stroke. J Nov Physiother Rehabil. 2025; 9(1): 007-012. Available from:
https://dx.doi.org/10.29328/journal.jnpr.1001065
DOI: 10.29328/journal.jnpr.1001065
Copyright license: © 2025 Herrera AS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CNS; Hydrogen; Dissociation; Protoporphyrin; Oxygen; Water
Insights into the Complexity of Paradoxical Antioxidants Behavior. And the Reasons for it’s almost Zero or no Effect on Stroke
Arturo Solís Herrera* , María del Carmen Arias Esparza and Sergey Shuckov
, María del Carmen Arias Esparza and Sergey Shuckov
Human Photosynthesis™ Study Center, Aguascalientes 20000, México
*Address for Correspondence: Arturo Solís Herrera, MD, PhD, Human Photosynthesis™ Study Center, Aguascalientes 20000, México, Email: [email protected]
Antioxidants are groups of compounds that neutralize free radicals and Reactive Oxygen Species (ROS) in the cell [1]. Antioxidant activity in food and beverages has become one of the most interesting features in the science community. These antioxidants provide protection against damage caused by free radicals played important roles in the development of many chronic diseases including cardiovascular diseases, aging, heart disease, anemia, cancer, and inflammation [2].
Antioxidants can be classified into two basic groups, synthetic and natural. In addition, the antioxidants can be classified as endogenous and exogenous according to their sources or as enzymatic or non-enzymatic according to their effects or water-soluble or lipid-soluble to their solubility [3].
Natural antioxidants are substances that exist in foods and prevent their reactions such as disruption, sourness, and color change. Natural antioxidants are generally derived from plant sources and their activity varies depending on plant species, diversity, extraction and/or processing methods, and growing conditions. They are found in microorganisms, in some animal tissues, and in almost all plants. Natural antioxidants, especially red, orange, and purple-colored fruits and vegetables, have a high antioxidant activity. Oranges, lemons, blueberries, strawberries, plums, prunes, red beans, broccoli flowers, and more have been proven to contain a high number of antioxidants and have been incorporated into many dietary menus. As the most important natural antioxidant groups, we can list tocopherols and tocotrienols, ascorbic acid, flavonoids, carotenoids, and phenolic acids [4].
So far, the human antioxidant defense system seems to be equipped with enzymatic scavengers such as Superoxide Dismutase (SOD), Catalase (CAT), and glutathione peroxidase; hydrophilic scavengers such as urate, ascorbate, Glutathione (GSH) and flavonoids and lipophilic radical scavengers such as tocopherols, carotenoids and ubiquinol. Non-enzymatic antioxidants contain subgroups, the main ones are vitamins, enzyme cofactors, minerals, peptides, phenolic acids, and nitrogen compounds [5].
E and C vitamins are the most important among vitamins as natural antioxidants. Vitamin C, which contains ascorbic acid and its oxidation product dehydroascorbic acid, has many biological activities in the human body. More than 85% of vitamin C in the human diet is provided by fruits and vegetables. Ascorbic acid can remove theoretically superoxide and hydroxyl radicals and may also regenerate a tocopherol [6]. It is important for the biosynthesis of collagen, carnitine, and neurotransmitters.
Vitamin E is a lipid-soluble vitamin that protects lipids against peroxidative damage with high antioxidant potential. Vitamin E is a chiral compound containing eight stereoisomers (a, b, c, d tocopherol and a, b, c, d tocotrienol). Only a-tocopherol is the most bioactive in humans [7]. Vitamin E is widely regarded as one of the most potent antioxidants. The antioxidant property is associated with the hydroxyl group in the aromatic ring, which gives hydrogen to neutralize free radicals or ROS [8]. Most of the tocopherols are a chemical class with vitamin E activity. Of these, a tocopherol is the main peroxyl radical remover in biological lipid phases, such as membranes or low-density lipoproteins [9].
Carotenoids (carotenes and xanthophylls) are yellow, orange, and red pigments found in many fruits and vegetables and are non-enzymatic natural antioxidants. The antioxidant effects of carotenoids are based on their singlet oxygen-quenching properties and their ability to capture peroxyl radicals. For example, lycopene, which is one of them, is known to be very protective, especially for prostate cancer. Lycopene’s main diet source is tomato, cooked tomato juice, and tomato sauce, which are more bioavailable than raw tomatoes.
Phenolic compounds are secondary metabolites of the plant but are also an important part of plant nutrients. Polyphenol-rich foods are widely studied due to their potential positive effects. Polyphenols have shown various positive bioactivities such as anticarcinogenic properties. Many compounds belong to the phenolic group. The most important ones in plants are flavonoids, phenolic acids, and stilbenes whose chemical structures can vary from very simple molecules to very complex molecules [10].
Flavonoids are a large group of low molecular weight polyphenolic substances and consist of benzo-c-pyrrole derivatives [11]. Flavonoids play an important role in various biological processes. They interact with several cellular targets in critical cell signaling pathways in the body to exhibit a variety of properties useful for human health. Flavonoids are a class of secondary metabolites that cover more than 10,000 structures [12]. Flavonoids are important antioxidants, especially because of their high redox potential; this allows them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. They also have the potential to chelate metal. Flavonoids are generally the most common phytochemicals in which these chemicals help protect the plant against UV light, fungal parasites, herbivores, pathogens, and oxidative cell damage. It has been associated with a decrease in the incidence of diseases such as cancer and heart disease with flavonoids when regularly consumed by humans [13].
So far, a summary of the literature on antioxidants, but they are publications whose authors continue to think that the human body takes the oxygen it requires for its functioning from the atmosphere that surrounds it. An idea that was born in the mid-eighteenth century, based on the operation of chimneys. So, they are concepts that will have to be rewritten for the most part.
Oxygen from the atmosphere and pulmonary alveolus
It is a deep-rooted dogma that the pulmonary alveolus is passively crossed by atmospheric oxygen, reaching the bloodstream and then being distributed to all the cells of the body.
But analyzing the history of this, we have to since 1771 when the oxygen in the atmosphere was discovered and characterized in the laboratory by Carl Wilhem Scheele, it was also found to be relatively scarce.
Lavoisier, based on Scheele’s work, proposed the role of oxygen in oxidizing metals and in respiration, but he wrongly believed that oxygen was taken up by the body during inhalation to allow slow combustion of organic substrates and that carbon dioxide was exhaled as a by-product.
100 years later (XIX Century) when the biochemistry of the human body was further studied, it was found that it had about 5 times more oxygen than the atmosphere. Since then, there has been a heated controversy about the origin of said oxygen [14].
Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Oxygen is highly electronegative, which creates a partial negative charge, which is enough to repel gaseous oxygen. Thereby, due to their high water content, the eukaryotic cells that make up the alveolar walls do not allow the passage of atmospheric oxygen, and even less by diffusion, which is the driving mechanism that is currently accepted. Supposedly, by the force of Brownian motion, gases are thought to pressure themselves through membranous barriers. On the other hand, gases such as oxygen form bubbles in liquids, shifting the question to how a bubble could diffuse through a continuous membrane [15].
Every few seconds we cycle between inspiration and expiration. During inspiration, our lungs draw in atmospheric gases, comprising not just oxygen (∼21%), but also nitrogen (∼78%). We also breathe argon (∼1%) and several trace gases [16]. Most of those inspired gases never make it past our lungs. Only oxygen manages apparently to pass from the alveoli (air sacs in the lungs) to the capillaries that envelop them. Nitrogen does not ordinarily pass, nor does argon, nor does it contain oxygen, even less so in the proportions required by the functioning of the body and the eukaryotic cell, which, in proportion, reaches almost five times more than that contained in the atmosphere [17].
For sure, oxygen deprivation leads quickly to suffocation, followed by death. Therefore, oxygen is critical for life. However, at extreme depths, oxygen is in short supply; yet fish manage to survive. If vertebrate life requires oxygen, then how do those fish make it? [18].
The performance of cell tasks, however, can be both dependent on and challenged by oxygen. Theoretically and contradictorily, Oxygen acts as the final electron acceptor for aerobic respiration but also participates in reactions to generate metabolites and structural macromolecules; recently, oxygen also has come to the fore for its signaling role in developmental programs in animals and plants. So, the oxygen needs of the cell are enormous, given the different roles that oxygen plays inside the cell.
During the Neoproterozoic and early Paleozoic eras, when atmospheric oxygen concentrations were substantially lower than today, evolution would have been almost impossible, unless the oxygen source of the prokaryotic and eukaryotic cell is the intracellular water that each of them contains.
This is not unreasonable as long as these living entities have molecules capable of dissociating water molecules, and melanin is the best example.
The unsuspected intrinsic property of melanin to dissociate the water molecule
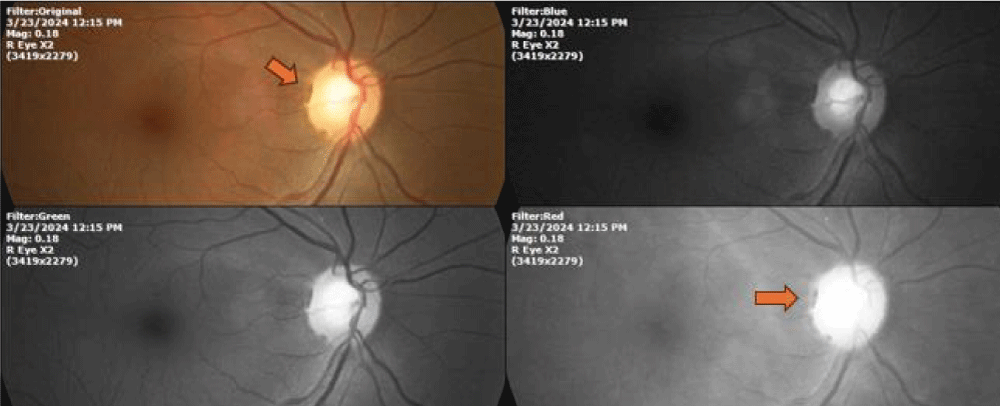
During an observational study carried out from 1990-2002, which included the ophthalmological records of 6000 patients, and whose working hypothesis was to establish possible correlations between the delicate morphology of the blood vessels that enter and exit through the tiny optic nerve in humans, whose average diameter is 1200 microns, with the three main causes of blindness in the world, which are: Age-related macular degeneration, diabetic retinopathy, and glaucoma (Figure 1), the continuous observation of the interaction between the different tissues around of the optic nerve, gradually led us to understand that the abundant melanin present in the area had more important actions than the simple role of sunscreen that is assigned to it to date.
Figure 1: Melanin is particularly abundant in the eye tissues, about 40% more than in the skin. The Orange arrow indicates a particularly visible accumulation of melanin.
After twelve years, and the ophthalmological records of six thousand patients included in this observational study [19], we were able to detect the unsuspected ability of the eukaryotic cell to transform the power of light into chemical energy, through the dissociation of water, as in plants.
Molecules derived from protoporphyrin IX, a molecule present in all living organisms [20] possess the intrinsic ability to irreversibly dissociate water molecules, using certain wavelengths of the visible spectrum. The reaction can be written as follows:
2H2O(liq) → 2H2(gas) + O2(gas)
Y están localizadas en diferentes estirpes celulares, tanto en animales como en plantas [21].
The only molecule present inside prokaryotic and eukaryotic cells, that can dissociate and reform water molecules, since it tolerates the toxicity of oxygen is melanin, and the reaction that occurs inside it, driven by electromagnetic radiation, visible and invisible; It can be written as follows:
2H2O(liq) → 2H2(gas) + O2(gas) → 2H2O(liq) + 4e-
This reaction (dissociation/reforming) can be considered within the very first reactions of life, that is: the dissociation of water, with which the liquid water, is contained inside the cell; It is the source from which the eukaryotic cell (and prokaryotic) obtains the oxygen (and hydrogen) it needs or requires to carry out the multitude of reactions that together make up what we call life.
The unsuspected ability of the cells of the human body to obtain the oxygen and hydrogen they constantly require by dissociating water, such as plants, overturns at least two dogmas:
- Glucose is not a source of energy, since its role is limited to being the universal precursor of any organic molecule, both in plants and animals.
- The oxygen that cells have inside them does not come from the atmosphere, but from the interior of each of the cells, just like CO2. The lung function is to expel CO2 that is constantly formed inside the cells that make up the human body. However, the alveoli do not have a mechanism to absorb or even promote the passage of atmospheric oxygen into the bloodstream. So, the gas lung exchange is not real, because the lung only expels CO2, but under any condition, CO2 is expelled in exchange for atmospheric O2. No way.
The histological descriptions in cases of Stroke refer to changes compatible with hypoxia. However, it is due to the fact that the generation of oxygen inside the cells, in this case, neurons, does not happen with the speed and accuracy that the cell requires, as is normal, which is manifested by edema and hemorrhage in any organ or tissue of the human body, followed by loss of form and function, and in chronic cases, disordered mitosis is a frequent finding.
Therefore, if the generation of oxygen and hydrogen inside the cells, in this case, neurons, is adequate, the body or tissue works well because it is very well done, but when this oxygen balance at the intracellular level becomes unbalanced, the tissues are proportionally altered. So, we must keep in mind that the oxygen present inside the cells or neurons comes from inside them, just like CO2.
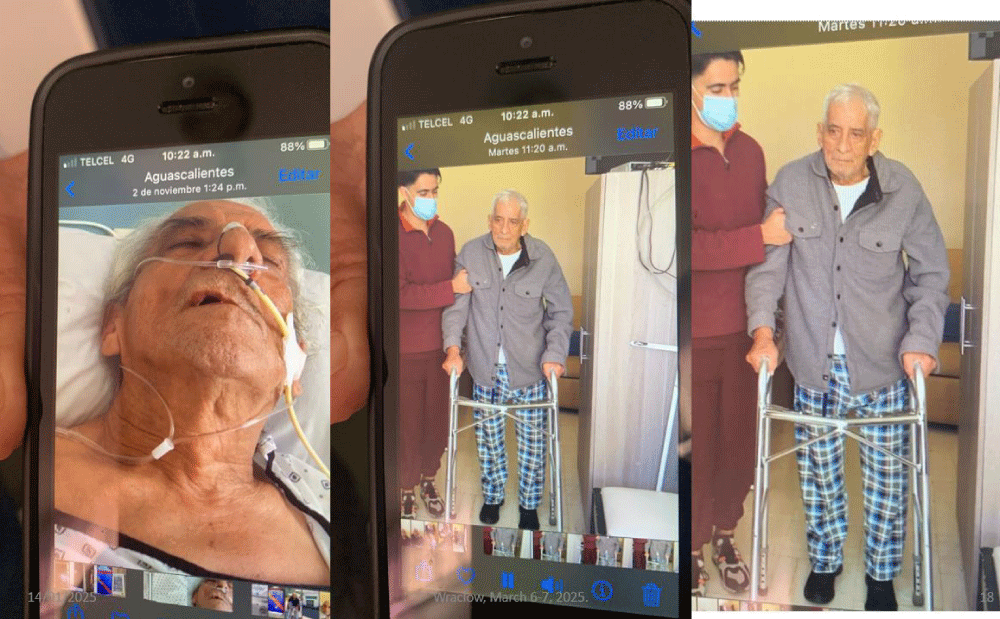
Hence, supplemental oxygen administered nasally or endotracheally does not represent a major advantage for the recovery of patients (Figure 2).
Figure 2: Sick in the ninth decade of life, with a diagnosis of cerebral vascular event (stroke), in coma, with poor prognostic. Note the delivery of oxygen through nasal prongs in the photograph on the left. Less than 72 hours after starting treatment with the therapeutic schemes developed in our study center, the patient was walking with help. (Right photograph).
The hitherto unknown importance of dissolved oxygen levels in water and their impact on neurodegenerative diseases
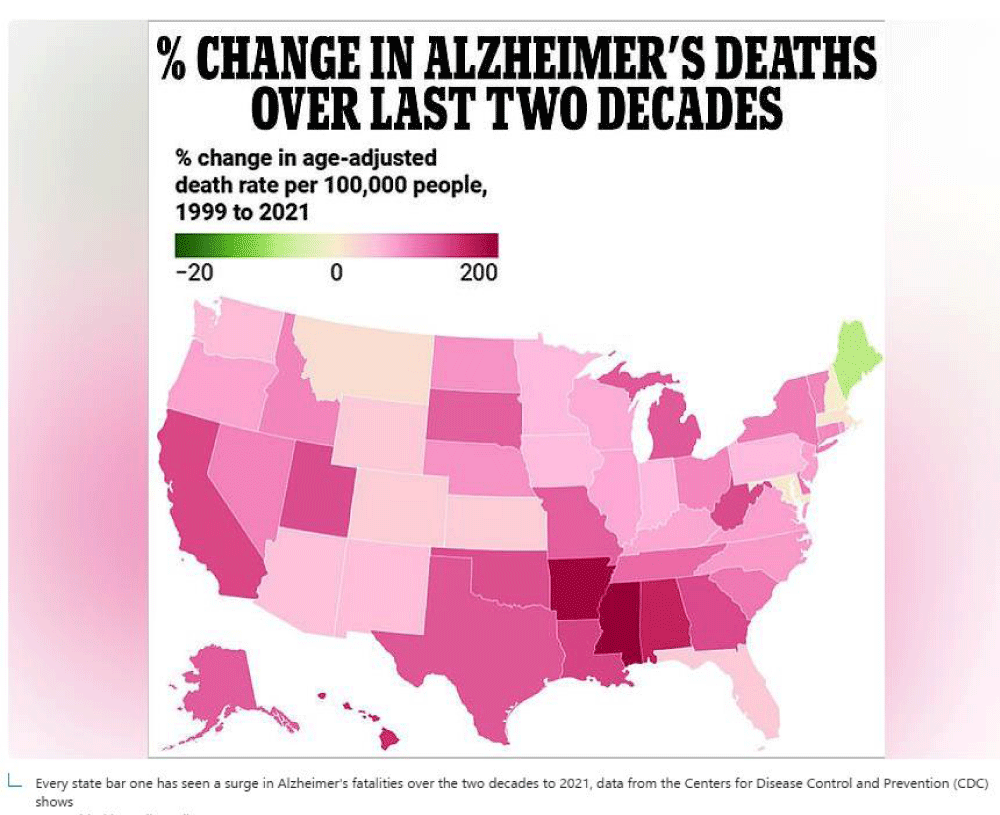
In a previously published article, we mentioned that the Stroke belt in the USA correlated with low levels of dissolved oxygen. That is: the lower the levels of dissolved oxygen in the water to which the population is exposed, the higher the incidence and prevalence of stroke, and vice versa.
An example of the higher the levels of dissolved oxygen in the drinking water to which the population is exposed, the lower the incidence and prevalence of stroke, as well as other neurodegenerative diseases such as Alzheimer’s Disease; is the U.S. state of Maine.
All the potable water in Georgetown, Maine comes from wells, of which nearly 75% are drilled into bedrock; the remaining 25% are dug wells. Bedrock wells are dependent upon the physical features in the bedrock, such as fractures, lineaments, and veins to provide water. There are four distinct aquifers in Georgetown which appear to operate independently of each other. The wells are generally six inches in diameter and were air rotary percussion or cable tool drilled. Around 75% of the wells have a yield of 2 gpm or greater, while 28% have a yield of 10 gpm or greater. Most of the bedrock wells are between 100 and 200 feet deep, and the water table is within 15 to 20 feet of the ground surface [23].
The dissolved oxygen content of Class A waters (in Maine) shall not be less than 7 ppm (7 mg/L) or 75% of saturation, whichever is higher, year-round. For the period from October 1 through May 14, in fish spawning areas, the 7-day mean dissolved oxygen concentration shall not be less than 9.5 ppm (9.5 mg/L), and the 1-day minimum dissolved oxygen concentration shall not be less than 8 ppm (8.0 mg/L) [23] (Figure 3).
Figure 3: The graph shows both the deleterious effect of low levels of dissolved oxygen in the water to which the population is exposed, shown in shades of carmine, red, and vice versa. That is: the apparent protective effect of high levels of dissolved oxygen in the water that is in contact with the population on epidemiologically important neurological diseases, such as Alzheimer's and Stroke disease. (Source of map: Daily Mail).
Comment
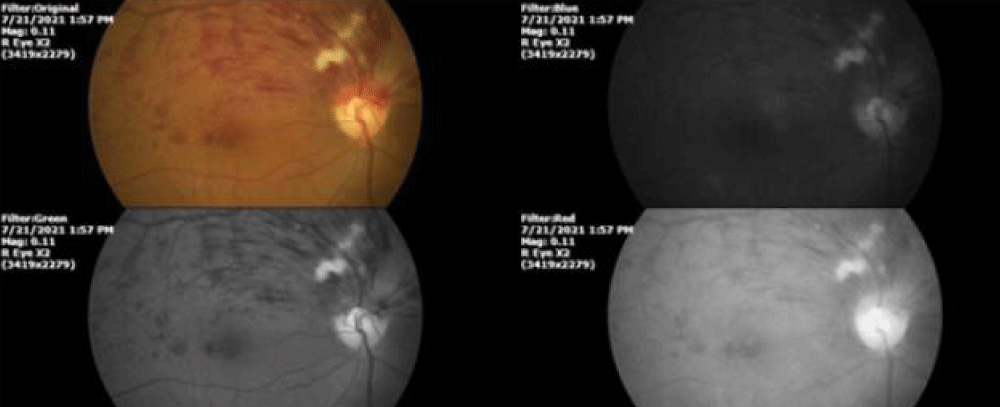
Stroke and acute myocardial infarction are primary global causes of mortality. Both are caused by alterations in blood flow. The severity of the histochemical alterations they produce was interpreted by the lack of blood supply that transported the essential oxygen. But now that we understand that each cell produces its own oxygen, restoring blood flow by physical or pharmacological means is not so imperative, since valuable oxygen comes from within the cell itself and not from the atmosphere, through the lung and then blood circulation. (Figures 4, 5).
Figure 4: Retinal vascular hypertension in a patient in the sixth decade of life, with a history of systemic arterial hypertension that is difficult to control. The involvement is in the superior temporal area, the vascular event is predominantly venous. After two months of little or no evolution despite orthodox treatments, the patient came to us. The photograph was taken during the first consultation on the day 02/21/2021.
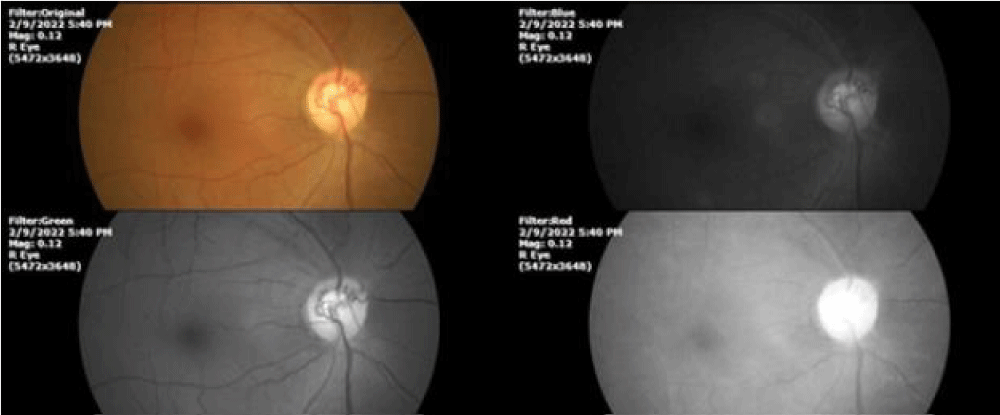
Figure 5: This photograph was taken on 02/09/2022. 19 months after starting the treatment based on our therapeutic schemes [24], the appearance of the initially affected tissues shows a surprising anatomical and functional recovery, which shows that the restoration of oxygen levels inside the cells is much more beneficial than the restoration of blood flow by physical or pharmacological means.
Statistical studies have shown that acute myocardial infarction is responsible for around 9 million deaths each year. Ischemic stroke and myocardial infarction have a significant role in global adult physical disabilities. So far, reperfusion has been considered vital for tissue recovery, but it may paradoxically, inadvertently increase damage through oxidative stress, inflammation, and cell death. Early reperfusion procedures were currently believed as the sole therapy to reduce infarct size. But the example of Figures 4,5 shows us that there are new treatments based on the hitherto unknown ability of eukaryotic and prokaryotic cells to dissociate the water molecule.
The incidence and prevalence of epidemiologically important diseases such as Alzheimer’s disease and stroke are inversely correlated with the levels of dissolved oxygen in drinking water to which the population is exposed. Thus, it is not only the water that is ingested, but also the water with which the dishes are washed, clothes are washed, bathed, house cleaned, food prepared, etc. Water with low levels of dissolved oxygen should not even be used to wash dishes.
The observation that our body does not take oxygen from the air that surrounds it but from the water that the cells are made up of has forced us to completely rewrite biology, medicine, and of course neurology.
The role of blood circulation does not include transporting atmospheric oxygen to the tissues, passing through the lungs and bloodstream. Hence, the administration of oxygen through the lungs does not mean an advantage in the treatment of patients affected by stroke.
On the other hand, the administration of antioxidants in a therapeutic way makes no sense, since the human body handles oxygen perfectly as it has been for the past six million years. Hence, the poor or no therapeutic effects of their administration.
The discovery of molecules capable of transforming the power of visible and invisible light into chemical energy that can be used by eukaryotic cells, as in plants, opens up a new panorama that will allow us to better and more deeply understand the very complex mysteries surrounding life, health, and disease, allowing us to offer greater possibilities of recovery and rehabilitation to patients affected by stroke. And other diseases that affect the function of the central nervous system, as well as the musculoskeletal system.
This work was supported by an unrestricted grant from Human Photosynthesis™ Research Center. Aguascalientes 20000, México.
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52:2522–2529. Available from: https://doi.org/10.1007/s13197-014-1396-5
- Vaibhav DA, Arunkumar W, Abhijit MP, Arvind S. Antioxidants as an immunomodulator. Int J Curr Pharm Res. 2011;1:8–10.
- Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–391. Available from: https://link.springer.com/article/10.1007/s00204-011-0774-2
- Shebis Y, Iluz D, Kinel-Tahan Y, Dubinsky Z, Yehoshua Y. Natural antioxidants: function and sources. Food Nutr Sci. 2013;4:643–649. Available from: http://dx.doi.org/10.4236/fns.2013.46083
- Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. Available from: https://doi.org/10.1016/j.fct.2012.09.021
- Podsedek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT. 2007;40:1–11. Available from: https://doi.org/10.1016/j.lwt.2005.07.023
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3614697/
- Peh HY, Tan WSD, Liao W, Wong WSF. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152–169. Available from: https://doi.org/10.1016/j.pharmthera.2015.12.003
- Fuchs-Tarlovsky V. Role of antioxidants in cancer therapy. Nutrition. 2013;29:15–21. Available from: https://doi.org/10.1016/j.nut.2012.02.014
- Jakobek L. Interactions of polyphenols with carbohydrates. Lipids Proteins Food Chem. 2015;175:556–567. Available from: https://doi.org/10.1016/j.foodchem.2014.12.013
- Leopoldini M, Russo N, Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. Available from: http://dx.doi.org/10.1016/j.foodchem.2010.08.012
- Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. Available from: https://doi.org/10.1016/j.plantsci.2012.07.014
- Ignat I, Volf I, Popa V. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. Available from: https://doi.org/10.1016/j.foodchem.2010.12.026
- Gjedde A. Diffusive insights: on the disagreement of Christian Bohr and August Krogh at the Centennial of the Seven Little Devils. Adv Physiol Educ. 2010;34(4):174-85. Available from: https://doi.org/10.1152/advan.00092.2010
- Da Conceicao M, Nemetz L, Rivero J, Hornbostel K, Lipscomb G. Gas Separation Membrane Module Modeling: A Comprehensive Review. Membranes (Basel). 2023;13(7):639. Available from: https://doi.org/10.3390/membranes13070639
- Glueckauf, E. The Composition of Atmospheric Air. In: Malone, T.F. (eds) Compendium of Meteorology. American Meteorological Society; 1951. Available from: https://www.scirp.org/(S(351jmbnt-vnsjt1aadkozje))/reference/referencespapers?referenceid=1295555
- Gibbs DJ, Holdsworth MJ. Every breath you take: New insights into plant and animal oxygen sensing. Cell. 2020;180:22–24. Available from: https://doi.org/10.1016/j.cell.2019.10.043
- Pollack GH. Is it oxygen, or electrons, that our respiratory system delivers? Med Hypotheses. 2024;192:111467. Available from: https://doi.org/10.1016/j.mehy.2024.111467
- Herrera AS. Towards a New Ophthalmic Biology and Physiology: The Unsuspected Intrinsic Property of Melanin to Dissociate the Water Molecule. MOJ Cell Sci Rep. 2017;4(2):00084. Available from: https://doi.org/10.15406/mojcsr.2017.04.00084
- Lv J, An X, Jiang S, Yang Y, Song G, Gao R. Protoporphyrin IX Stimulates Melanogenesis, Melanocyte Dendricity, and Melanosome Transport Through the cGMP/PKG Pathway. Front Pharmacol. 2020;11:569368. Available from: https://doi.org/10.3389/fphar.2020.569368
- Herrera AS. The Biological Pigments in Plants Physiology. Agric Sci. 2015;6:1262-1271. Available from: http://dx.doi.org/10.4236/as.2015.610121
- Georgetownme.com. Georgetown Water Quality Re-evaluation Final Report [Internet]. 2014 Sep. Available from: https://www.georgetownme.com/wp-content/uploads/2014/09/georgetown_water_quality_re-evaluation_final_report.pdf
- Code of Federal Regulations (CFR) 131.43 (up to date as of 1/10/2025) Maine. Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-131/subpart-D/section-131.43
- Solís H, del Carmen A, Eugenia Solís A. Retinal Vein Occlusion Treated with QIAPI 1™: Report of a Case. JOJ Ophthalmol. 2022;9(3):555762. Available from: https://juniperpublishers.com/jojo/pdf/JOJO.MS.ID.555762.pdf