More Information
Submitted: July 15, 2024 | Approved: July 22, 2024 | Published: July 23, 2024
How to cite this article: Parizotto NA, Ferraresi C. Enhancing Physiotherapy Outcomes with Photo-biomodulation: A Comprehensive Review. J Nov Physiother Rehabil. 2024; 8(2): 031-038. Available from: https://dx.doi.org/10.29328/journal.jnpr.1001061
Copyright License: © 2024 Parizotto NA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Enhancing Physiotherapy Outcomes with Photobiomodulation: A Comprehensive Review
Nivaldo Antonio Parizotto1* and Cleber Ferraresi2
1Biomedical Engineering, University Brasil, São Paulo, Brazil
2Department of Physical Therapy, Federal University of São Carlos, São Carlos, Brazil
*Address for Correspondence: Nivaldo Antonio Parizotto, Biomedical Engineering, University Brasil, São Paulo, Brazil, Email: [email protected]; [email protected]; nivaldo.parizotto universidadebrasil.edu.br
Physiotherapy treatments employ complex approaches tailored to the patient’s diagnosis. Exercise is the primary strategy to enhance rehabilitation processes for most individuals. However, electrophysical agents, such as Photobiomodulation (PBM), that utilize specific wavelengths of light to penetrate tissues and stimulate cellular activity, can modulate various biological processes and may improve physiotherapy outcomes. This non-invasive treatment can reduce pain and inflammation, promote tissue repair, and accelerate tissue healing. Currently, PBM has numerous applications, including pain and inflammation treatment, wound healing (such as diabetic foot ulcers, pressure ulcers, post-surgery wounds, and skin grafts in burn injuries), and the management of musculoskeletal disorders (such as arthritis, tendinopathies, muscle injuries, and spinal disorders). It is also utilized to improve muscle performance and recovery in rehabilitation and sports. Additionally, transcranial PBM has shown promise in enhancing neurorehabilitative processes by facilitating the recovery of cognitive and motor functions in various types of lesions. The safety and efficacy of this treatment allow it to be incorporated alongside regular exercises and manual therapies as an adjunctive treatment, potentially enhancing outcomes in different areas of rehabilitation.
Photobiomodulation (PBM) is a treatment method utilizing non-ionizing visible and near-infrared wavelengths, resulting in photophysical and photochemical events without causing thermal damage. The therapeutic application of the correct dose of light from lasers, light-emitting diodes (LEDs), and broadband lights with filters elicit biological responses based on PBM principles, known as Photobiomodulation Therapy (PBMT) [1].
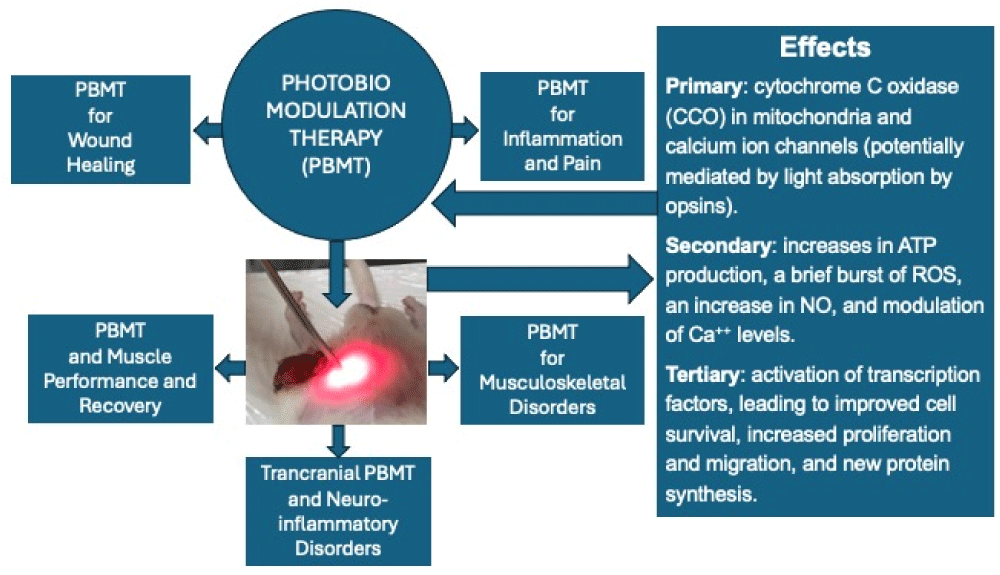
The mechanisms involved in the interaction of light with biological tissues primarily involve chromophores, such as cytochrome C Oxidase (CCO) in mitochondria and calcium ion channels (potentially mediated by light absorption by opsins). Secondary effects of photon absorption include increases in Adenosine Triphosphate (ATP) production, a brief burst of Reactive Oxygen Species (ROS), an increase in Nitric Oxide (NO), and modulation of calcium levels. Tertiary effects involve the activation of a wide range of transcription factors, leading to improved cell survival, increased proliferation and migration, and new protein synthesis (Figure 1). Notably, there is a pronounced biphasic dose response, where low light levels have stimulating effects, while high light levels have inhibitory effects [1].
Figure 1: Scheme describing the main physiological effects of Photobiomodulation (PBM), including cellular and molecular effects. Based on these effects, some of the main clinical applications (since there are others) of PBM described in this text are presented.
PBM is distinguished by its non-invasive, painless, and easy-to-perform method, with a well-proven degree of effectiveness, both in terms of its mechanisms and clinical outcomes [2]. Additionally, extensive information on the peripheral and central mechanisms of pain modulation by PBM has been elucidated [3,4]. We can include therapeutic effects on pain control and inflammation, wound healing, in musculoskeletal disorders, to improve muscle performance in rehabilitation and sports, and enhance the rehabilitation process of neuro-inflammatory disorders using the transcranial approach of PBM (Figure 1).
Pain and inflammation
Pain is often associated with inflammation, and PBM has significant effects on both acute and chronic inflammatory processes [2]. PBM interferes with inflammatory reactions, modulating them effectively [4]. Pain is a complex and multifactorial experience that can be acute or chronic, significantly impacting individuals’ quality of life. Studies have shown that PBM can reduce pain through various mechanisms, including modulating inflammation, increasing adenosine ATP production, and promoting the release of endorphins.
A study by Chow, et al. [5] highlighted the effectiveness of PBM in reducing pain in musculoskeletal conditions, demonstrating that the application of red and near-infrared light significantly improved pain and functionality in patients with chronic neck pain. Additionally, a clinical trial conducted by Enwemeka, et al. [6] showed that PBM effectively reduced pain in patients with knee osteoarthritis, promoting healing and decreasing the need for analgesic medications.
Inflammation is the body’s essential biological response to injury or infection, but chronic inflammation can lead to various diseases. PBM has been shown to modulate the inflammatory response effectively, accelerating the healing process and reducing the signs and symptoms of inflammation. Yamany and El-Sayed [7] conducted a study using PBM to treat inflammation in patients with rheumatoid arthritis, resulting in a significant reduction in inflammatory markers and an improvement in joint mobility. Furthermore, a review by Hamblin [1] elucidated the mechanisms by which PBM reduces inflammation, including the modulation of macrophage activity and the decreased expression of pro-inflammatory cytokines. PBM modulates the expression of inflammatory mediators, such as cytokines, and influences the activity of macrophages, which are essential cells in the immune response. Reducing inflammation and oxidative stress are key aspects of relieving pain and promoting healing [1].
PBM in wound healing
PBM utilizing Low-Level Laser Therapy (LLLT) and Light-Emitting Diodes (LEDs), has garnered substantial attention for its efficacy in enhancing wound healing processes. Numerous randomized clinical trials, systematic reviews, and meta-analyses have explored the mechanisms and clinical outcomes associated with PBM in wound management. This review highlights recent findings and discusses the potential of PBM in promoting wound healing.
PBM promotes wound healing through several mechanisms. It stimulates cellular processes by ATP production and modulating Reactive Oxygen Species (ROS). These effects enhance cellular proliferation, migration, and differentiation, which are crucial for tissue repair. Additionally, PBM promotes angiogenesis, and collagen synthesis, and modulates inflammatory responses, leading to accelerated and more effective wound healing [1].
Recent Randomized Controlled Trials (RCTs) have demonstrated the benefits of PBM in wound healing across various types of wounds, including diabetic foot ulcers, pressure ulcers, and surgical wounds. For example, Minatel, et al. [8] conducted an RCT evaluating the effect of PBM on diabetic foot ulcers. The study included 60 patients who were randomly assigned to receive either PBM or a placebo treatment. Results indicated a significant reduction in wound size and an improvement in healing rates in the PBM group compared to the placebo group. This study concluded that PBM could be an effective adjunctive therapy for managing diabetic foot ulcers.
Karimpour, et al. [9] demonstrated that the combination of autologous platelet gel (APG) with PBM improves healing time, wound grade, pain reduction, and granulation tissue formation in diabetic foot ulcers. Their study highlighted that various PBM modalities, involving distinct probes and wavelengths, exhibit the potential to enhance tissue perfusion, expedite healing, and impede wound progression, thus reducing the need for invasive interventions. PBM combined with APG has emerged as a valuable tool for augmenting wound healing, mitigating inflammation, and preventing amputation, representing a compelling therapeutic option for diabetic foot ulcers.
Recent systematic reviews and meta-analyses have synthesized data from various RCTs to provide comprehensive insights into the efficacy of PBM in wound healing. Tchanque-Fossuo, et al. [10] performed a meta-analysis of four studies involving 131 participants who met the inclusion criteria. The endpoints evaluated included ulcer size and time to complete healing, with follow-up periods ranging from 2 to 16 weeks. Each study was assessed for evidence level and graded according to the Oxford Center for Evidence-based Medicine Levels of Evidence Grades of Recommendation criteria. Despite limitations such as small sample sizes (N < 100), unclear allocation concealment, and short follow-up periods, the review concluded that PBM by LLLT demonstrates significant potential as a portable, minimally invasive, and cost-effective modality for treating diabetic foot ulcers.
Santos, et al. [11] reviewed thirteen RCTs and found that PBM using LLLT was effective in treating diabetic foot ulcers. They identified three RCTs that reported a significant reduction in ulcer size, which was confirmed through meta-analysis. Their findings indicated that using wavelengths in the red range (632.8 nm to 685 nm), with a fluence of 50 mW/cm²,3 to 6 J/cm², and irradiation for 30 to 80 seconds, three times weekly for one month, was beneficial for patients with diabetic foot ulcers. Similarly, Wang, et al. [12] in a Cochrane review found that phototherapy (PBM) might increase the proportion of completely healed wounds during follow-up and reduce wound size in diabetic patients, though there was no evidence that it improved quality of life.
A systematic review by Huang, et al. [13] assessed the efficacy of PBM in treating pressure ulcers. The review included 12 RCTs with a total of 482 patients and found that PBM significantly enhanced wound healing, reduced inflammation, and promoted tissue regeneration in pressure ulcer patients. The authors recommended PBM as a promising adjunct therapy for managing pressure ulcers, though the quality of evidence was rated as very low according to the GRADE system.
In the field of plastic surgery, Meyer, et al. [14] evaluated PBM therapy using transcutaneous irradiation of vascular targets starting three days post-surgery. They used transcutaneous PBM each session lasted 30 min, using 660 nm (red), energy 180 J. For all groups, the therapy started with daily use for seven days followed the interval use of three times a week until completed 21 days. The study demonstrated significant improvements in heart rate at rest, systolic and diastolic blood pressure, and peripheral oxygen saturation in the active therapy groups. In the McGill Scale evaluation, the mean total score showed a more accentuated drop in the groups that used PBM. ILIB may have prevented a more significant evolution of fibrosis levels, however, no changes were observed in the evaluation of sleep and anxiety [14].
Kazemikhoo, et al. [15] evaluated the effects of PBM by LLLT on the healing of skin-grafted areas in burn patients. Their study found that PBM is a safe and effective method for improving graft survival and the wound healing process, while also decreasing the rate of wound dehiscence in patients with deep burn ulcers.
Taradaj, et al. [16] examined the efficacy of PBM at different wavelengths (940 nm, 808 nm, and 658 nm) for treating pressure ulcers. The primary endpoint of this trial included both the percentage reduction in the ulcer surface area and the percentage of completely healed wounds after one month of therapy (ulcer healing rate). The secondary endpoint was the ulcer healing rate at the follow-up evaluation (3 months after the end of the study). In total, 72 patients with stage II and III pressure ulcers received laser therapy once daily, 5 times per week for 1 month, using a (GaAlAs) diode laser with a maximum output power of 50 mW and continuous radiation emission. Three different wavelengths were used for the laser treatment: 940 nm, 808 nm, and 658 nm. An average dose of 4 J/cm2 was applied. In the placebo group, the laser device was turned off. This study found that laser therapy at 658 nm was effective in reducing pressure ulcers, whereas the wavelengths of 808 nm and 940 nm did not show significant effects.
Recent evidence supports the efficacy of PBM in enhancing wound healing across various wound types. The mechanisms through which PBM promotes wound healing, such as increasing ATP production, reducing inflammation, and stimulating collagen synthesis, are well-documented. However, further large-scale, high-quality RCTs are needed to establish standardized protocols for optimal dosages, treatment durations, and wavelengths. Finally, PBM is a promising therapeutic modality for wound healing, offering a non-invasive, effective, and safe treatment option. Its integration into clinical practice could significantly improve outcomes for patients with chronic and acute wounds.
PBM in musculoskeletal disorders
Musculoskeletal disorders encompass a broad range of conditions, including arthritis, tendinitis, muscle injuries, and spinal disorders, which can lead to chronic pain, inflammation, and decreased mobility. PBM has emerged as a promising therapeutic approach for these conditions due to its ability to accelerate tissue repair and modulate inflammatory processes.
Osteoarthritis, a common degenerative joint condition, is characterized by pain and stiffness. A recent study by Huang, et al. [17] explored the effects of PBM on patients with knee osteoarthritis. The study found that an eight-week PBM treatment regimen led to a significant reduction in pain and an improvement in joint function. The authors concluded that PBM may serve as an effective and non-invasive alternative to traditional treatments for osteoarthritis. Ahmad, et al. [18] published a systematic review and meta-analysis of knee osteoarthritis demonstrating that the association of exercises with PBM done by low-level laser and high-intensity laser can improve the results.
Tendinitis, or tendinopathy, involves inflammation and sometimes degeneration of tendon collagen and other fibers, which can be debilitating and challenging to treat. Leal-Junior, et al. [19] evaluated the effectiveness of PBM in patients with Achilles tendinitis. Their study demonstrated that PBM significantly reduced pain and accelerated functional recovery compared to a control group that did not receive therapy. Their study supports PBM as a viable option for the rehabilitation of tendon injuries. In a randomized controlled trial comparing PBM alone and the usual care (UC) in plantar fasciitis, Kets, et al. [20] show effects on pain not different between PBMT groups. PBMT in both treatment groups also improved function more than the UC group, again with the improvement occurring within the first three weeks. Pain and function improved during the three weeks of PBMT plus UC and remained stable over the following three weeks. Improvements were sustained through six months in the PBMT plus UC groups. Level II- RCT or Prospective Comparative Study. To investigate the effects of PBM on plantar fasciitis, Dos Santos, et al. [21] carried out a systematic review and meta-analysis pointing out the best dosages and treatment techniques for this clinical condition.
Muscle injuries, frequently encountered in athletes and physically active individuals, can also benefit from PBM. Anders, et al. [22] investigated the application of PBM in an animal model of muscle injury. Their findings revealed that PBM accelerated muscle healing and reduced inflammatory markers. This study indicates that PBM may be beneficial for managing muscle injuries in both sports and clinical settings.
De Toni, et al. [23] show the results using ultrasound and laser that had a greater tendency to ameliorate pain with a high-size effect. Between groups, there was a significant difference in post-treatment of the right trapezius in the exercise and photobiomodulation groups. The photobiomodulation group showed pre and post-intervention differences in the left trapezius maximum onset. The conclusion was that the interventions with photobiomodulation, ultrasound, and exercise help pain, function, and muscular activation in seamsters with neck pain.
In a triple-blinded randomized clinical trial, Navarro-Ledesma, et al. [24] carried out an interesting study using a whole-body PBM applied in patients with fibromyalgia resulted in a significant reduction in pain and an improvement in quality of life in those participants after receiving 4 weeks of treatment. Furthermore, psychological factors such as kinesiophobia and self-efficacy were also improved showing that treatment can be considered efficient in this chronic pain syndrome.
The beneficial effects of PBM are attributed to its ability to modulate the expression of inflammatory cytokines, reduce inflammation, and decrease oxidative stress, all of which are critical factors in pain management and tissue repair. In clinical practice, PBM for musculoskeletal disorders should be applied with specific parameters, including the wavelength of light, dosage, duration of treatment, and treatment area. Research suggests that wavelengths in the range of 600-1200 nm are most effective for deep tissue penetration and achieving optimal therapeutic effects. Personalizing treatment based on individual patient characteristics—such as skin phototype, stage of inflammation, and the patient’s nutritional and hydration status—is essential for maximizing the benefits of PBM.
In summary, PBM has emerged as a promising therapy for musculoskeletal disorders, offering a non-invasive and effective approach for reducing pain, and inflammation, and promoting tissue repair. As a complementary treatment, PBM has the potential to significantly enhance clinical outcomes for patients with a variety of musculoskeletal conditions.
PBM and muscle performance and recovery
The use of LT and/or LEDs, recently recognized as PBM, for enhancing muscle performance during exercise and skeletal muscle recovery post-exercise has attracted attention, including from the International Olympic Committee [25]. In a brief historical context, one of the initial studies exploring PBM for muscle performance enhancement and post-exercise muscle recovery was conducted by Craig, et al. [26,27]. These authors reported no effect of PBM using red (660 nm) and infrared (950 nm) wavelengths on reducing delayed onset muscle soreness (DOMS) induced by repeated eccentric contractions of the elbow flexors in young men.
Nearly a decade following these pioneering clinical trials, two animal model studies (in vivo) were published investigating the effects of PBM on muscle performance in rats [28,29]. The first study applied PBM to the hind limbs of rats after each training session over 5 weeks, aiming to accelerate muscle recovery and modulate lactate dehydrogenase (LDH) enzyme activity (linked to lactate production and oxidation - energy metabolism) during treadmill training [28]. The second study [29] applied PBM to the anterior tibial muscle of rats before a muscle fatigue protocol induced by electrical stimulation. PBM was applied prior to the fatigue protocol to reduce muscle fatigue and muscle damage as indicated by creatine kinase (CK) blood levels. These two animal model studies laid the groundwork for the two primary approaches currently recognized for applying PBM to skeletal muscles to enhance exercise performance and accelerate post-exercise recovery [30-32]:
- Approach 1: Irradiation of skeletal muscles as preconditioning therapy (before exercise).
- Approach 2: Irradiation of skeletal muscles as muscle recovery therapy (irradiation after exercise).
Since 2008, with the simultaneous publication of two randomized clinical trials [33,34], many randomized clinical trials and animal model studies have been published with the same purpose: to investigate the effects of PBM as preconditioning or applied after exercise to enhance muscle performance and accelerate post-exercise recovery [30]. A series of studies involving athletes, non-athletes, and healthy individuals, and using various study designs (blinded, non-blinded, randomized, crossover, with sham therapy) in controlled settings such as research laboratories have been published to examine the acute effects of PBM on muscle fatigue and prevention of muscle damage (lower CK activity in the bloodstream) during strenuous exercises [30]. Additionally, in 2011, the first study combining the effects of photobiomodulation with a strength training program (chronic effects) was published [35]. In 2015, research moved from controlled laboratory environments, and the first randomized clinical trial with athletes in the field was conducted during the Brazilian Volleyball Championship. In this study, athletes from one of the participating teams in the championship were irradiated with LED on their lower limbs as preconditioning 40-60 minutes before each of the 4 official games of the championship in which PBM was tested. This study demonstrated a dose-response, or therapeutic window, of PBM when applied as a preconditioning for preventing muscle injury (lower CK activity in the bloodstream) in professional volleyball athletes during official games [36].
The main effects of PBM on muscle performance and post-exercise recovery reported to date include resistance to fatigue (increased number of repetitions or increased time of muscle contraction until exhaustion), peak torque or force enhancement (typically analyzed by an isokinetic dynamometer and maximum repetition tests), and prevention of muscle damage [lower Creatine Kinase (CK) or LDH activity measured in the bloodstream] as reported in previous reviews and meta-analyses [32,37]. The potential mechanisms behind these main effects have been previously described [30] along with other mechanisms confirmed in more recent randomized clinical trials, such as increased skeletal muscle hypertrophy [37,38] and modulation of gene expression related to protein synthesis/degradation, inflammation, and oxidative stress [37]. Additionally, other in vitro (39) and in vivo studies [39,40] have confirmed the purported increase in mitochondrial metabolism of skeletal muscle cells (previously observed only in hepatocytes or tumor cell lines), as well as increased glycogen [40] and ATP synthesis [39,40] when PBM is applied as preconditioning [39] or when applied after exercise, promoting better outcomes compared to preconditioning [40].
Recently, González-Muñoz, et al. [41] demonstrated that based on current scientific literature, the utilization of PBM therapy before and/or after exercise appears to be efficacious for enhancing sports performance in both strength training and cardiovascular exercise. This therapy facilitates the mitigation of muscle damage, reduces blood lactate levels, enhances oxygen uptake, and alleviates post-exercise muscle soreness. Consequently, these effects contribute to improved athletic performance and consequently superior competitive outcomes. Their findings suggest that integrating PBMT into athletes’ training regimens may enhance outcomes and expedite recovery processes, thereby augmenting their overall sports performance.
Transcranial PBM and neuro-inflammatory disorders
Transcranial Photobiomodulation (tPBM) has emerged as a promising non-invasive technique for enhancing brain function and treating various neurological conditions. By utilizing Near-Infrared Light (NIR), typically in the range of 600-1000 nm, tPBM penetrates the scalp and skull to interact with cortical and subcortical neurons, leading to a cascade of beneficial physiological effects, that improve the neurorehabilitation with exercises or functional training.
The primary mechanism of tPBM involves the absorption of light by mitochondrial chromophores, notably CCO. This absorption enhances mitochondrial respiration and ATP production, which in turn promotes neuronal function and survival. Additionally, tPBM can modulate the production of ROS and induce the expression of neuroprotective genes and proteins. These cellular and molecular effects contribute to improved neuroplasticity, synaptogenesis, and neuroprotection [42,43]. All these effects have important action on brain repair using rehabilitation techniques.
Recent studies have investigated the role of tPBM in cognitive enhancement among both healthy individuals and those with cognitive impairments. In a randomized controlled trial, Barrett and Gonzalez-Lima [44] demonstrated that tPBM applied to the prefrontal cortex significantly improved sustained attention and working memory in healthy adults. Similarly, Chao [45] found that tPBM enhanced executive function and information processing speed in older adults with Mild Cognitive Impairment (MCI). These findings suggest that tPBM may serve as a non-pharmacological intervention to bolster cognitive function in aging populations.
In addition to cognitive enhancement, tPBM has shown potential in treating mood disorders such as depression and anxiety. A study by Cassano, et al. [46] reported that tPBM targeting the dorsolateral prefrontal cortex (DLPFC) resulted in significant reductions in depressive symptoms among patients with major depressive disorder (MDD). Furthermore, Liu, et al. [47] observed that tPBM alleviated anxiety symptoms in patients with generalized anxiety disorder (GAD), highlighting its anxiolytic effects. These outcomes are attributed to tPBM’s ability to modulate neurochemical pathways and enhance cortical excitability.
tPBM is also being explored for its neurorehabilitative potential in stroke and Traumatic Brain Injury (TBI) patients. A recent pilot study by Naeser, et al. [48] found that tPBM improved cognitive and motor functions in chronic stroke patients, potentially by promoting cortical reorganization and neurogenesis. In TBI patients, Mitrofanis and Jeffery [49] demonstrated that tPBM facilitated recovery by reducing neuroinflammation and oxidative stress. These studies underscore the potential of tPBM as an adjunctive therapy in neurorehabilitation protocols.
The application of tPBM in neurodegenerative diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) has also gained attention. A study by Berman, et al. [50] revealed that tPBM improved cognitive performance and reduced beta-amyloid plaques in an Alzheimer’s disease (AD) mouse model. Similarly, a clinical trial by El Khoury, et al. [51] demonstrated that tPBM enhanced motor function and decreased motor symptoms in PD patients. These effects are likely mediated through tPBM’s neuroprotective properties and its ability to enhance mitochondrial function and reduce oxidative stress.
The safety profile of tPBM has been extensively studied, with most research indicating minimal side effects when appropriate dosages and protocols are followed [52]. Future research should focus on large-scale randomized controlled trials to further elucidate the optimal parameters for different conditions and to establish standardized treatment protocols. Additionally, exploring the combinatory effects of tPBM with other therapeutic modalities (like exercises and another electrophysical agent such as neuromuscular electrostimulation) could open new avenues for enhancing its efficacy.
Overall, transcranial photobiomodulation has demonstrated significant potential in enhancing cognitive function, alleviating mood disorders, supporting neuro-rehabilitation, and managing neurodegenerative diseases. The mechanisms underlying these effects are rooted in improved mitochondrial function, enhanced neuroplasticity, and modulation of neurochemical pathways. As research progresses, tPBM could become a cornerstone in the non-invasive treatment of various neurological conditions, offering a safe and effective therapeutic approach.
The existing body of evidence supports the efficacy of Photobiomodulation (PBM) as a valuable therapeutic tool for a range of pathophysiological conditions [5,53]. Studies have demonstrated that PBM offers a cost-effective alternative for routine clinical use in various applications, including those discussed in this review. While this review has highlighted several key applications of PBM, there remain numerous additional fields where its potential benefits could be explored. Notably, emerging areas such as women’s health, respiratory conditions, neurological disorders, and dermatological treatments warrant further investigation.
PBM has shown good cost-effectiveness and therapeutic benefits that make this therapy a promising alternative in clinical practice [54]. Current evidence not only elucidates the mechanisms underlying PBM’s effects but also supports its application across diverse clinical scenarios, including pain management, inflammation reduction, and functional improvement in various physiological systems [55]. The comprehensive understanding of these mechanisms, combined with PBM’s established effectiveness, underscores its potential as a versatile therapeutic instrument.
Future research should aim to expand on the applications of PBM, particularly in the aforementioned fields, and refine treatment protocols to optimize therapeutic outcomes. By exploring combinatory approaches with other modalities and conducting large-scale, randomized controlled trials, the full scope of PBM’s therapeutic potential can be realized. As the field continues to evolve, PBM holds promise as a cornerstone in non-invasive therapy, offering a safe and effective solution for a wide range of medical conditions.
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337-361. Available from: https://doi.org/10.3934/biophy.2017.3.337.
- Alqualo-Costa R, Rampazo EP, Thome GR, Perracini MR, Liebano RE. Interferential current and photobiomodulation in knee osteoarthritis: A randomized, placebo-controlled, double-blind clinical trial. Clin Rehabil. 2021;35(10):1413-1427. Available from: https://doi.org/10.1177/02692155211012004.
- Pigatto GR. Involvement of the descending endogenous pain modulation system in antinociceptive control by light-emitting diode therapy. Doctorate Thesis – Post Graduation Program in Biotechnology in Regenerative Medicine and Medical Chemistry – University of Araraquara, Brazil; 2020.
- Pigatto GR, Silva CS, Parizotto NA. Photobiomodulation therapy reduces acute pain and inflammation in mice. J Photochem Photobiol B. 2019;196:111513. Available from: https://doi.org/10.1016/j.jphotobiol.2019.111513.
- Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomized placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908. Available from: https://doi.org/10.1016/s0140-6736(09)61522-1.
- Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;22(4):323-329. Available from: https://doi.org/10.1089/pho.2004.22.323.
- Yamany MS, El-Sayed WF. Effect of low-level laser therapy on neurovascular function of diabetic peripheral neuropathy. J Adv Res. 2013;4(6):459-466.
- Minatel DG, Frade MAC, França CM, Enwemeka CS. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg Med. 2019;41(6):433-441. Available from: https://doi.org/10.1002/lsm.20789.
- Karimpour S, Amirmotamed MH, Rashno F, Tahmasebinia F, Keramatinia A, Fathabadi FF, et al. Unveiling Therapeutic Potential: A Systematic Review of Photobiomodulation Therapy and Biological Dressings for Diabetic Foot Ulcers. J Lasers Med Sci. 2023;14. Available from: https://doi.org/10.34172/jlms.2023.49.
- Tchanque-Fossuo CN, Ho D, Dahle SE, Koo E, Li CS, Isseroff RR, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen. 2016;24(2):418-426. Available from: https://doi.org/10.1111/wrr.12399.
- Santos CM dos, Rocha RB, Hazime FA, Cardoso VS. A Systematic Review and Meta-Analysis of the Effects of Low-Level Laser Therapy in the Treatment of Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2021;20(3):198-207. Available from: https://doi.org/10.1177/1534734620914439.
- Wang HT, Yuan JQ, Zhang B, Dong ML, Mao C, Hu D. Phototherapy for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017;6. Available from: https://doi.org/10.1002/14651858.cd011979.pub2.
- Huang J, Chen J, Xiong S, Huang J, Liu Z. The effect of low-level laser therapy on diabetic foot ulcers: A meta-analysis of randomized controlled trials. Int Wound J. 2021;18(6):763-776. Available from: https://doi.org/10.1111/iwj.13577.
- Meyer PF, Maia RR, de Morais Carreiro E, da Silva RMV, Farias SLQ, Picariello F, et al. Analysis of modified ilib therapy in patients submitted to plastic surgery. Lasers Med Sci. 2024;39(1):110. Available from: https://link.springer.com/article/10.1007/s10103-024-04057-4.
- Kazemikhoo N, Vaghardoost R, Dahmardehei M, Mokmeli S, Momeni M, Nilforoushzadeh MA, et al. Healing process after skin gragft surgery in burned patients (A randomized Clinical Trial). J Lasers Med Sci. 2018;9(2):139-143. doi: 10.15171/jlms.2018.26. Available from: https://doi.org/10.15171%2Fjlms.2018.26.
- Taradaj J, Halski T, Kucharzewski M, Urbanek T, Halska U, Kucio C. Effect of Laser Irradiation at Different Wavelengths (940, 808, and 658 nm) on Pressure Ulcer Healing: Results from a Clinical Study. Evid Based Complement Alternat Med. 2013;2013:960240. doi: 10.1155/2013/960240. Available from: https://doi.org/10.1155/2013/960240.
- Huang YY, Chen AC, Carroll JD, Hamblin MR. Low-level laser therapy (LLLT) for treatment of osteoarthritis. Photomed Laser Surg. 2022;40(1):14-21.
- Ahmad MA, A Hamid MS, Yusof A. Effects of low-level and high-intensity laser therapy as adjunctive to rehabilitation exercise on pain, stiffness and function in knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. 2022;114:85-95. Available from: https://doi.org/10.1016/j.physio.2021.03.011
- Leal-Junior ECP, Vanin AA, Miranda EF, de Carvalho PD, Dal Corso S, Bjordal JM. Effect of photobiomodulation therapy (PBMT) on Achilles tendinitis in athletes: a randomized, double-blind, placebo-controlled trial. Lasers Med Sci. 2021;36(2):251-258.
- Ketz AK, Anders J, Orina J, Garner B, Hull M, Koreerat N, et al. Photobiomodulation Therapy Plus Usual Care Is Better than Usual Care Alone for Plantar Fasciitis: A Randomized Controlled Trial. Int J Sports Phys Ther. 2024;19(1):1438-1453. Available from: https://doi.org/10.26603/001c.90589.
- Dos Santos SA, Sampaio LM, Caires JR, Fernandes GHC, Marsico A, Serra AJ, et al. Parameters and Effects of Photobiomodulation in Plantar Fasciitis: A Meta-Analysis and Systematic Review. Photobiomodul Photomed Laser Surg. 2019;37(6):327-335. Available from: https://doi.org/10.1089/photob.2018.4588.
- Anders JJ, Moges H, Wu X. Photobiomodulation for the treatment of muscle injuries: a study on animal models. J Biophotonics. 2023;17(5)
- De Toni MM, Duarte RS, das Neves LMS, Diefenthaeler F, Fonseca MCR, Barbosa RI, et al. Physiotherapeutic approach in seamstresses with neck pain: A single-blind, randomized clinical trial. J Bodyw Mov Ther. 2022;31:90-96. doi: 10.1016/j.jbmt.2022.03.008. Available from: https://doi.org/10.1016/j.jbmt.2022.03.008.
- Navarro-Ledesma S, Carroll J, Burton P. Short-Term Effects of Whole-Body Photobiomodulation on Pain, Quality of Life and Psychological Factors in a Population Suffering from Fibromyalgia: A Triple-Blinded Randomised Clinical Trial. Pain Ther. 2023;12:225-239. Available from: https://doi.org/10.1007/s40122-022-00450-5.
- Hainline B, Derman W, Vernec A, Budgett R, Deie M, Dvorak J, et al. International Olympic Committee consensus statement on pain management in elite athletes. Br J Sports Med. 2017;51(17):1245-1258. Available from: https://doi.org/10.1136/bjsports-2017-097884.
- Craig JA, Barlas P, Baxter GD, Walsh DM, Allen JM. Delayed-onset muscle soreness: lack of effect of combined phototherapy/low-intensity laser therapy at low pulse repetition rates. J Clin Laser Med Surg. 1996;14(6):375-380. Available from: https://doi.org/10.1089/clm.1996.14.375.
- Craig JA, Barron J, Walsh DM, Baxter GD. Lack of effect of combined low intensity laser therapy/phototherapy (CLILT) on delayed onset muscle soreness in humans. Lasers Surg Med. 1999;24(3):223-230. Available from: https://doi.org/10.1002/(sici)1096-9101(1999)24:3%3C223::aid-lsm7%3E3.0.co;2-y.
- Vieira W, Goes R, Costa F, Parizotto N, Perez S, Baldissera V, et al. Enzymatic adaptation of LDH in rats subjected to aerobic training on a treadmill and low-intensity laser. Braz J Physiother. 2006;10:205-211. Available from: https://doi.org/10.1590/S1413-35552006000200011.
- Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC, Muscara MN, Aimbire F, et al. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol (1985). 2006;101(1):283-288. Available from: https://doi.org/10.1152/japplphysiol.01318.2005 .
- Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012;1(4):267-286. Available from: https://doi.org/10.1515/plm-2012-0032.
- Ferraresi C, Huang YY, Hamblin MR. Photobiomodulation in human muscle tissue: an advantage in sports performance? J Biophotonics. 2016;9(11-12):1273-1299. Available from: https://doi.org/10.1002/jbio.201600176.
- Vanin AA, Verhagen E, Barboza SD, Costa LOP, Leal-Junior ECP. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: a systematic review and meta-analysis. Lasers Med Sci. 2018;33(1):181-214. Available from: https://doi.org/10.1007/s10103-017-2368-6.
- Gorgey AS, Wadee AN, Sobhi NN. The effect of low-level laser therapy on electrically induced muscle fatigue: a pilot study. Photomed Laser Surg. 2008;26(5):501-506. Available from: https://doi.org/10.1089/pho.2007.2161.
- Leal Junior EC, Lopes-Martins RA, Dalan F, Ferrari M, Sbabo FM, Generosi RA, et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26(5):419-424. Available from: https://doi.org/10.1089/pho.2007.2160.
- Ferraresi C, de Brito Oliveira T, de Oliveira Zafalon L, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, et al. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci. 2011;26(3):349-358. Available from: https://doi.org/10.1007/s10103-010-0855-0.
- Ferraresi C, Dos Santos RV, Marques G, Zangrande M, Leonaldo R, Hamblin MR, et al. Light-emitting diode therapy (LEDT) before matches prevents increase in creatine kinase with a light dose response in volleyball players. Lasers Med Sci. 2015;30(4):1281-1287. Available from: https://doi.org/10.1007/s10103-015-1728-3.
- Ferraresi C, Bertucci D, Schiavinato J, Reiff R, Araujo A, Panepucci R, et al. Effects of Light-Emitting Diode Therapy on Muscle Hypertrophy, Gene Expression, Performance, Damage, and Delayed-Onset Muscle Soreness: Case-control Study with a Pair of Identical Twins. Am J Phys Med Rehabil. 2016;95(10):746-757. Available from: https://doi.org/10.1097/phm.0000000000000490.
- Baroni BM, Rodrigues R, Freire BB, Franke Rde A, Geremia JM, Vaz MA. Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur J Appl Physiol. 2015;115(3):639-647. Available from: https://doi.org/10.1007/s00421-014-3055-y.
- Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med Sci. 2015;30(4):1259-1267. Available from: https://doi.org/10.1007/s10103-015-1723-8.
- Ferraresi C, Parizotto NA, Pires de Sousa MV, Kaippert B, Huang YY, et al. Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J Biophotonics. 2015;8(9):740-54. Available from: https://doi.org/10.1002/jbio.201400087
- González-Muñoz A, Cuevas-Cervera M, Pérez-Montilla JJ, Aguilar-Núñez D, Hamed-Hamed D, Aguilar-García M, et al. Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare. 2023;11(7):938. Available from: https://doi.org/10.3390/healthcare11070938.
- Salehpour F, Majdi A, Pazhuhi M, Ghasemi F, Khademi M, Pashazadeh F, et al. Transcranial Photobiomodulation Improves Cognitive Performance in Young Healthy Adults: A Systematic Review and Meta-Analysis. Photobiomodul Photomed Laser Surg. 2019;37(10):635-643. Available from: https://doi.org/10.1089/photob.2019.4673.
- Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neurosci Lett. 2018;665:36-40.
- Chao LL. Transcranial photobiomodulation improves cognitive performance and functional connectivity in older adults with mild cognitive impairment: a pilot study. J Alzheimers Dis. 2019;71(3):765-774.
- Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV, Buchheit CL. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Photomed Laser Surg. 2019;37(10):617-625.
- Liu TC, Huang CM, Huang LT, Chen CH, Chen YC. Transcranial photobiomodulation for the treatment of generalized anxiety disorder: a pilot study. Photobiomodul Photomed Laser Surg. 2020;38(2):89-95.
- Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2020;38(3):170-179.
- Mitrofanis J, Jeffery G. Does photobiomodulation influence ageing? Aging (Albany NY). 2018;10(9):2224-2225. doi: 10.18632/aging.101556. Available from: https://doi.org/10.18632/aging.101556.
- Berman MH, Halper JP, Nichols TW, Gerber J, Sanner J. Photobiomodulation-induced changes in beta-amyloid levels in the brain of an Alzheimer's disease mouse model: a pilot study. Photobiomodul Photomed Laser Surg. 2018;36(11):584-590.
- El Khoury H, Mitrofanis J, Henderson LA. Exploring the effects of transcranial photobiomodulation in Parkinson's disease: a preliminary study. J Parkinsons Dis. 2019;9(3):503-510..
- Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Opt. 2017 Jan;19(1):013003. Available from: https://doi.org/10.1088/2040-8986/19/1/013003.
- Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514-530. Available from: https://doi.org/10.7326/m16-2367.
- Sobral AP, Sobral SS, Campos TM, Horliana AC, Fernandes KP, Bussadori SK, Motta LJ. Photobiomodulation and myofascial temporomandibular disorder: Systematic review and meta-analysis followed by cost-effectiveness analysis. J Clin Exp Dent. 2021;13(7). Available from: https://doi.org/10.4317/jced.58084.
- González-Muñoz A, Perez-Montilla JJ, Cuevas-Cervera M, Aguilar-García M, Aguilar-Nuñez D, Hamed-Hamed D, Pruimboom L, Navarro-Ledesma S. Effects of Photobiomodulation in Sports Performance: A Literature Review. Appl Sci. 2023;13:3147. Available from: https://doi.org/10.3390/app13053147.
- Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, et al. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem Photobiol. 2015;91(2):411-416. Available from: https://doi.org/10.1111/php.12397.
- Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516-533. Available from: https://doi.org/10.1007/s10439-011-0454-7.