More Information
Submitted: June 25, 2024 | Approved: July 02, 2024 | Published: July 03, 2024
How to cite this article: Rajanchellappa S, Khurana D, Sinha AGK, Kathirvel S, Kumar A, et al. Development and Evaluation of a mHealth app - (ReMiT-MS app) for Rehabilitation of Individuals with Relapsing-remitting Multiple Sclerosis - A Mixed Methods, Pragmatic Randomized Controlled Trial - Study Protocol. J Nov Physiother Rehabil. 2024; 8: 022-030.
DOI: 10.29328/journal.jnpr.1001060
Copyright License: © 2024 Rajanchellappa S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Multiple sclerosis; mHealth app; Exercise; Rehabilitation; Self-management; Pragmatic trial
Development and Evaluation of a mHealth app - (ReMiT-MS app) for Rehabilitation of Individuals with Relapsing-remitting Multiple Sclerosis - A Mixed Methods, Pragmatic Randomized Controlled Trial - Study Protocol
Solaiyan Rajanchellappa1, Dheeraj Khurana2*, AGK Sinha3, Soundappan Kathirvel4, Ashok Kumar5 and Rajni Sharma6
1Department of Physical and Rehabilitation Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
2Department of Neurology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3Department of Physiotherapy, Punjabi University, Patiala Punjab, India
4Department of Community Medicine and School of Public Health, Postgraduate Institute of Medical Education and Research, Chandigarh, India
5Department of National Institute of Nursing Education, Postgraduate Institute of MedicalEducation and Research, Chandigarh, India
6Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India
*Address for Correspondence: Dheeraj Khurana, Department of Neurology, Postgraduate Institute of Medical Education and Research, Chandigarh India, Tel: +91 7087009695, Email: khurana.dheeraj@pgimer.edu.in; dherajk@yahoo.com
Background: Delaying or slowing functional loss is a valuable goal of Multiple Sclerosis (MS) rehabilitation. The mHealth app-based exercise rehabilitation intervention is expected to overcome barriers related to routine care of MS. Due to the ubiquitous presence of smartphones, they offer an excellent opportunity for remote monitoring, scheduled interaction with experts, and instruction for exercise in a home environment. Challenges in MS routine care include forgotten rehabilitation steps, limited access to local MS experts, and internal barriers such as low health literacy, mobility limitations, and fatigue, alongside external obstacles like service availability and transport costs.
Objectives: To develop a mHealth app that is user-centered and context-specific for rehabilitation of MS symptoms, and to evaluate its clinical and cost effectiveness in individuals with RRMS.
Methods: The proposed research will be conducted in two phases; the first phase (Phase 1a) will be focused on the development of mHealth app content (ReMiT-MS app trial I). The pilot phase (Phase 1b), where a prototype of the application will be designed, and its usability will be evaluated. Finally, in the second phase (Phase 2), the clinical and cost-effectiveness of the ReMiT-MS app for the rehabilitation of individuals with RRMS will be evaluated (ReMiT-MS app trial II).
Conclusion: The findings of this proposed trial may provide a telerehabilitation platform for individuals with RRMS in a resource-limited setting and establish a low-cost healthcare delivery model. In addition, the results of this research work might open a new window in healthcare delivery in India and similar settings.
Trial registration: CTRI/2022/09/045266 [Registered on 06/09/2022]
Background and rationale
Multiple Sclerosis (MS) is a chronic, immune-mediated, inflammatory, and demyelinating disease of the central nervous system and is the most common non-traumatic cause of chronic neurological dysfunction in adults [1]. The global burden of MS is estimated at 2.8 million cases (common among young adults; male-female ratio: 1:2) [2] and India is no exception. In the last few years, the number of reported MS cases has been increasing in India due to increased availability, accessibility, and affordability of magnetic resonance imaging (MRI) and more practicing neuro physicians with a roughly estimated prevalence of 5–10 per 100,000 individuals [3,4].
Delaying or slowing functional loss becomes a valuable goal and mainstay of the management of MS, and physical rehabilitation is an important aspect of achieving this goal. Due to the chronic and progressive nature of MS, individuals with MS need long-term rehabilitation and continuous monitoring from rehabilitation professionals. In routine rehabilitation practice (unsupervised home-based rehabilitation) individuals with MS are usually instructed to perform exercises regularly as recommended by the physiotherapists and receive written protocols for self-management at home. Depending on the progression of symptoms, the individuals are advised to revisit the hospital for exercise modification.
However, compliance with the home exercise program is a challenge, and individuals with MS often return for follow-up visits when their condition deteriorates. Lack of a constant caregiver [5], forgotten rehabilitation steps, and limited access to local MS experts [6] are major obstacles to implementing adequate exercise rehabilitation. In addition, various internal barriers, such as low health literacy, limited mobility, lack of motivation, fatigue, and related issues, and external barriers such as availability and accessibility of services and transport costs also contribute [7]. All these factors pose significant challenges for delivering routine MS care, especially adherence to exercise rehabilitation. Therefore, education about self-management and remote monitoring of individuals with MS may be viable solutions for the rehabilitation of MS [8]. To address these barriers, easily accessible and available technology-based solutions like mobile health applications (mHealth app) can be adapted for providing telerehabilitation and monitoring. In recent years, mobile apps with a variety of objectives such as improving cognition [9], fatigue [10-12], dexterity [13] and physical performance [6]; have been developed and evaluated its effectiveness and feasibility for MS, and studies have reviewed the already existing apps in databases [14] and app stores [15,16]. These review studies have highlighted limitations of the existing apps in terms of their functionality, their supply and demand mismatch, their failure to meet some of the self-management needs, and the gap in the collaboration of patients and health care providers in app development. Additionally, limited access in app stores, cost, low empowerment, and lack of scientific evidence were the limitations of existing mHealth applications for MS identified by the study authors.
Hence, keeping the limitations of already existing mHealth applications in mind, a user-centered and context-specific mHealth app for symptom management by physical rehabilitation measures needs to be developed and evaluated for clinical and cost-effectiveness in individuals with the most prevalent and less disease-burden type of MS especially relapsing-remitting multiple sclerosis (RRMS).
Objectives
1. To develop a mHealth app that is user-centered and context-specific for the rehabilitation of individuals with RRMS.
2. To evaluate its clinical and cost-effectiveness in individuals with RRMS.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting guidelines are used to report study protocol [17].
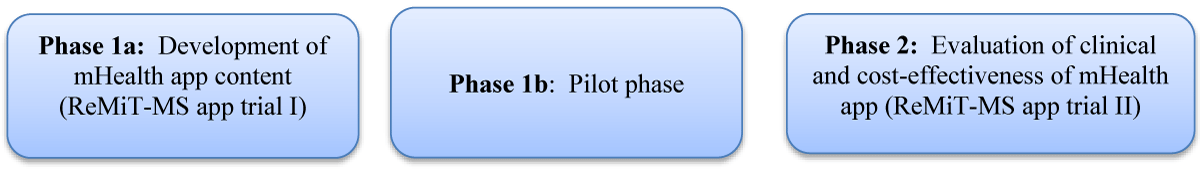
Trial design overview (Figure 1)
Figure 1: Study overview
ReMiT-MS (rehabilitation using mHealth app-based intervention for MS) app trial will be conducted in two phases: The first phase (Phase 1a) will be focused on the development of mHealth app content, which will be based on literature evidence and understanding health challenges and rehabilitation practices of individuals with RRMS using a mixed-methods approach (ReMiT-MS app trial I). The pilot phase (Phase 1b), where a prototype of the application will be designed, and its usability will be evaluated using the mHealth application usability questionnaire (MAUQ). Finally, in the second phase (Phase 2), the clinical and cost-effectiveness of the ReMiT-MS app for the rehabilitation of individuals with RRMS will be evaluated through a mixed method, pragmatic parallel-group, open-labeled, randomized controlled trial (ReMiT-MS app trial II).
Study setting: Patients will be recruited from the multiple sclerosis clinic of the Department of Neurology, PGIMER Chandigarh, India.
Detailed methods of phase 1: Development and piloting of mHealth-app-A mixed-methods study design (ReMiT-MS- I)
The study will be conducted in two sub-phases: Preliminary phase (phase 1a): which will develop the mHealth app content. Pilot phase (phase 1b): will assess the usability of the mHealth application-ReMiT-MS app.
Phase 1a: Development of mHealth app content: The objective of phase 1a is to identify the problems related to health and rehabilitation practices in individuals with RRMS to develop the mHealth app.
The following steps will be taken to achieve this objective:
1. Conduct a systematic review of the existing literature on RRMS, including symptoms, and the role of physical rehabilitation in managing those symptoms. This review can help to identify the most common symptoms experienced by individuals with RRMS, as well as the types of physical rehabilitation interventions that are most effective for managing those symptoms.
2. Understanding Health Challenges and Rehabilitation Practices of individuals with RRMS using a mixed-methods approach:
- Quantitative: A cross-sectional study will be conducted to identify the most prevalent physical complaints experienced by individuals with RRMS; to assess the effectiveness and limitations of current rehabilitation practices used by this population; and to gather valuable insights that will guide the rationale for the development of a mHealth application specifically tailored to effectively address the challenges faced by individuals with RRMS, with the ultimate goal of empowering them to manage their condition and improve their overall quality of life. The study will use the total enumeration technique to study all eligible individuals visiting the MS clinic for six months from the first recruited patient. To be eligible for the study, individuals with RRMS (as per McDonald 2017 criteria [18] diagnosed for more than six months and under treatment, must meet the following inclusion criteria: a) age 18 to 50, b) EDSS score 0 to 4.5, c) clinical stability or free of relapse for at least one month before study enrolment, d) MMSE score equal to or more than 24, and e) willingness to follow study procedures. Exclusion criteria include not being on any disease-modifying therapy, pregnancy and lactation, a diagnosis of progressive forms of MS, chronic illness like TB, HIV, and Hepatitis B & C and any chronic diseases that affect physical activities.
- Qualitative: In-depth interviews will be conducted to evaluate the lived experiences and rehabilitation practices, among individuals with RRMS. Participants will be selected using convenience sampling, and the sample size will be decided based on principles of redundancy (information saturation). Only individuals who have consented to voice recording will be recruited. Data analysis will be done using Collaizzi's method.
Prototype of the mHealth app content
o Based on the results of steps 1 and 2, the prototype of the mHealth app content, i.e., rehabilitation intervention protocol will be developed. The rehabilitation protocol for individuals with RRMS will emphasize patient education and self-management information in an audiovisual format. It will comprise exercise rehabilitation approaches to improve muscle strength, and coordination/balance, reduce muscle spasticity, and education on fatigue and bladder management. The tentative framework of mHealth-app content is given in Table 1. The validity of the mHealth app content, i.e., rehabilitation intervention protocol, will be assessed by 10-12 experts in the field of MS rehabilitation. As per the expert opinion, modifications to the content will be made. After that, videos of these procedures will be developed. The finalized videos will be transferred into a mobile app compatible with smartphones on the Android platform with the help of a professional technical expert. At the end of phase 1a (preliminary phase), a preliminary app will be developed for testing and refinement (Pilot study).
| Table 1: Tentative framework of ReMiT-MS app content. | ||
| Feature content | Description | Purpose |
| 1) Information provision | Importance of physical rehabilitation in MS | Education and Information |
| 2) Video demonstration with audio | Exercise rehabilitation approaches: • Detailed video demonstration with instructions for each exercise according to the symptoms • Progressively more difficult exercises to improve or maintain the physical status of health |
Demonstration of exercises. |
| 3)Audio explanation | Fatigue and bladder management | Education and Information |
| 4)Recorded exercises by participants | Recordings were maintained on the calendar to review each session of exercises that were performed | Monitoring |
| 5) Motivational statements | To improve adherence and reminder messages for exercises | Motivation |
| 6)Exercise diary | Weekly record to allow reflection on exercise performance | Self- monitoring |
Phase 1b: Pilot phase: This phase of the study involves the pilot phase, which aims to assess the usability of the mHealth application. The study will involve a small group of individuals with RRMS who meet the inclusion and exclusion criteria mentioned in the study.
During the pilot phase, participants will be given access to the mHealth app and will practice using it for a month. The usability of the application will be evaluated using the mHealth application usability questionnaire (MAUQ), for the interactive mHealth app (patient version); which is a validated questionnaire used to assess the usability of mHealth apps specifically on mobile devices. The questionnaire will consist of 21 items related to ease of use, interface and satisfaction, and usefulness of the mHealth app. All items will be rated on a 7-point Likert scale, with 1 representing strongly disagree and 7 strongly agree. A higher score will indicate the higher usability of the app [19]. The results of the pilot phase will be used to identify any usability issues and areas for improvement in the mHealth app. Based on the results obtained from the pilot phase, additional modifications will be made to the content and workflow of the app for the final version ensuring that the final version of the mHealth app is user-friendly, effective, and meets the needs of individuals with RRMS for symptom management.
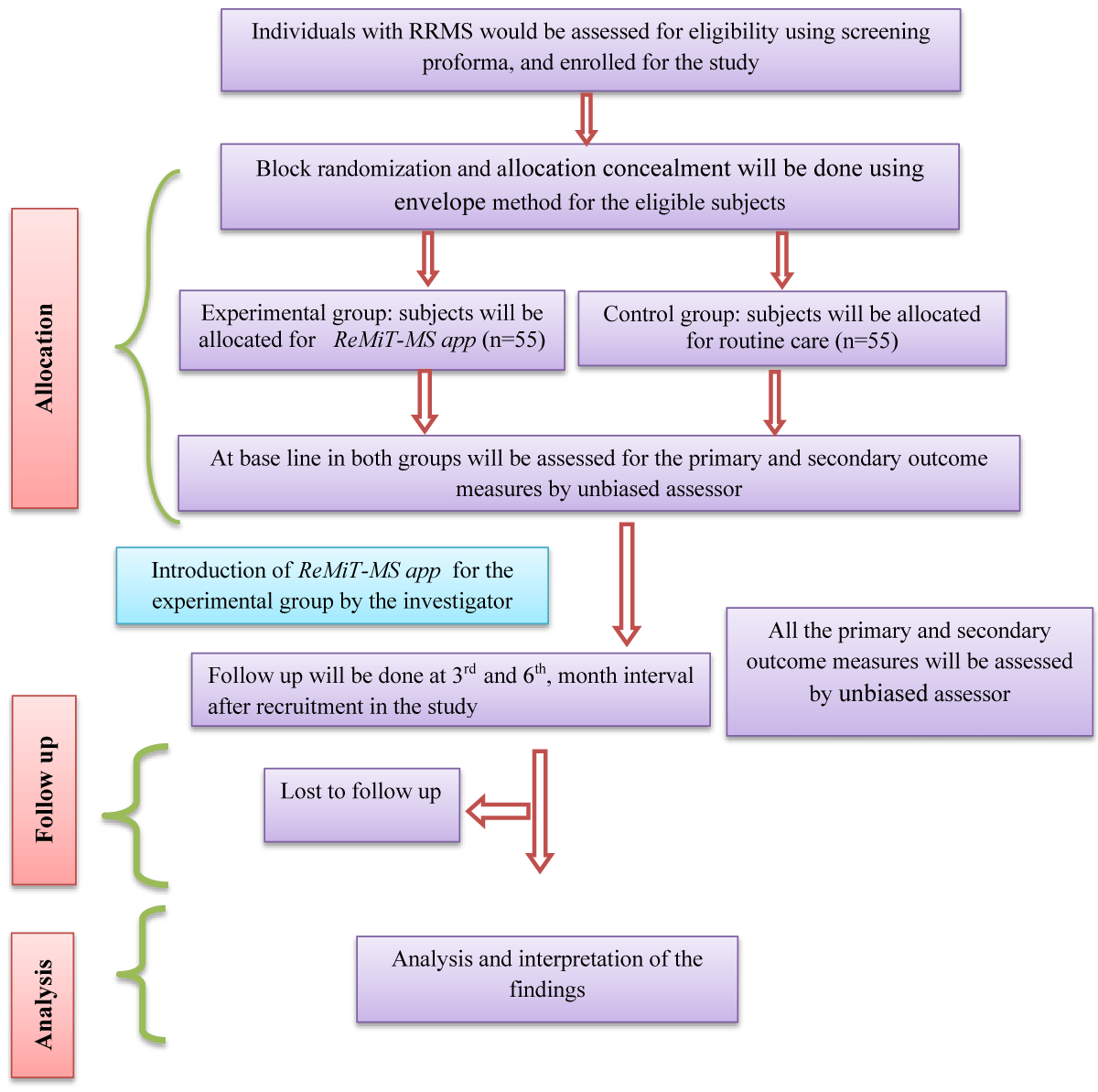
Phase 2: Evaluation of clinical and cost-effectiveness of mHealth-app – A mixed method, pragmatic parallel, open-labeled, randomized controlled trial. (ReMiT-MS II) (Figure-2)
Figure 2: Consort flow diagram of phase 2.
Study objective: To evaluate the clinical and cost-effectiveness of the ReMiT-MS app as a user-centered and context-specific tool for the rehabilitation of MS symptoms in individuals with RRMS.
Study design: This phase of the study would be a Pragmatic Prospective Randomized Open labeled with a Blinded End-point assessment (PROBE) design; the individuals with relapsing MS will be randomized into 2 study groups (control – routine care group and experimental – ReMiT-MS -app-based exercise rehabilitation group).
Sample size: Pragmatic exercise compared to usual care has shown a difference of 3 points in overall quality of life (MSQOL-54), (A.M. Carter et al. / Contemporary Clinical Trials 35 (2013) 40–47) [20]. In our study, we are comparing comprehensive mHealth app-based exercise rehabilitation vs routine care where an anticipated difference is 8-10 points. A sample size of 46 subjects would be required in each arm to show a difference of at least 10 points between the groups, at 80% power and 5% significance level. Considering a 20% attrition rate, we will recruit a total of 110 subjects, i.e., 55 subjects per arm.
Randomization and blinding: Individuals with RRMS (as per McDonald 2017 criteria) will be screened and recruited from the MS clinic of our center. After signing the informed consent forms, the study participants will be randomized into two groups, such as the routine care group (n = 55) and the ReMiT-MS app-based exercise rehabilitation group (n = 55). To achieve equal size, permuted block randomization with varying block sizes (2, 3, and 4) will be used. Allocation concealment will be done using the opaque sealed envelope method. The block randomization and allocation concealment will be done by an independent researcher. This will be an open-blinded study, only the assessors are blinded to the treatment cluster of the participants.
Eligible criteria for phase 2: To be eligible for the study, individuals with RRMS (as per McDonald 2017 criteria [18]) diagnosed for more than six months and under treatment, must meet the following inclusion criteria: a) age 18 to 50, b) EDSS score 0 to 4.5, c) clinically stable or relapse-free for at least one month before enrolment, d) MMSE score more than 24 e) willingness to follow study procedures and f) Individuals must have a smartphone with internet facility and should be competent as well as willing to use an app as health care tool g) Must have a 20/70 or better in at least one eye on Visual Acuity Test (VAT). Exclusion criteria include not being on any disease-modifying therapy, pregnancy and lactation, a diagnosis of progressive forms of MS, chronic illness like TB, HIV, and Hepatitis B & C, and any chronic diseases that affect physical activities. Individuals who have visual impairment significantly affecting visibility will be excluded.
Interventions: Two in-home exercise programs will be evaluated in this study: the control group will receive routine care (exercise handout and logbook), while the experimental group will receive ReMiT-MS app-based exercise rehabilitation over six months; assessing effects in the 3rd and 6th months. Individuals will receive directions for an in-home exercise program from an experienced physiotherapist and researcher during the enrollment period of the study.
Exercise guideline
The guidelines of the US National MS Society recommendations for exercise and physical activity will be adapted [21]. Guidelines specify ≥150 min/week of exercise and/or ≥150 min/week of lifestyle physical activity. Exercise prescription, a variety of sets and repetitions to be completed for every exercise will be prescribed one by one for each participant, depending on their baseline assessments, and will be modified and progressed according to individual abilities, preferences, and safety. All modifications will be recorded. Both groups will be advised to carry out similar exercise rehabilitation measures, including exercises and physical activity depending on their baseline assessment, for at least five days per week (30 min a day) for six months.
Control group (routine care)
The control group will receive routine care based on patient education by self-management, which includes exercise handouts and a logbook. The handouts will describe the exercise steps with pictures and progressively difficult exercises. Individuals with RRMS and their caregivers will be trained to undertake self-management for long-term care. They will be advised to maintain a logbook to document the exercise regimen and the time taken to accomplish each exercise. There will be no restrictions for hospital revisits for exercise modification.
Experimental group (ReMiT-MS app-based rehabilitation)
The experimental group (ReMiT-MS app) will receive the app-based rehabilitation intervention. This app will be used online and free of cost, available in Hindi and English. The installation of the app on the participant’s phone will be facilitated by the investigator. Subsequently, the investigator will provide the username and password for the app login, along with operational instructions and an explanation of its features. Successful training will be defined as three or more errorless attempts to retrieve any required part of the intervention from the app. Individuals who are using the app for the first time must first register. Then, they are advised to complete a symptom-assessment questionnaire to evaluate symptoms, leading to the opening of the symptom-based exercise intervention page within the app. This section will include textual files and audio-visual demonstrations explaining each exercise, progressively increasing in difficulty. The app also contains features such as an exercise diary, educational resources, and contact details of the investigator. The app will be linked to a mobile/website administrator module accessible to the investigator, allowing for performance tracking and protocol compliance monitoring. Additionally, a member of the research team will conduct monthly phone calls and bi-weekly reminder messages to reinforce and encourage the benefits of exercise.
Concomitant care permitted during the trial
During the study, the individuals in the experimental group will be advised to follow the ReMiT-MS app-based exercise rehabilitation, and any queries regarding the exercises will be sorted out via phone calls with the investigator to avoid frequent hospital revisits. There will not be any restriction to the control group to visit the hospital for exercise modification. Further, there will not be any restrictions for hospital visits for consultations with a physician in both groups.
Outcome measures: An unbiased assessor who is unaware of the randomization and group assignment will evaluate all outcome measures (baseline, post-intervention at 3rd and 6th month of recruitment). The detailed participant timeline is given in Table 2.
| Table 2: Participant timeline. | ||
| Phases | Methodology | Duration & follow-up |
| Patient screening` Patient information sheet about the study |
||
| Phase 1 (Development phase) |
Preliminary phase Informed consent Quantitative – cross-sectional study – using data collection form. Qualitative – descriptive study – by semi-structured interview© |
The preliminary phase will be conducted over six months. This phase is a brief one-time nonexperimental study design, thus no follow-up is required. |
| Pilot phase Informed consent To assess the user-friendliness and assist in the final design of the app. |
One-month follow-up. | |
| Phase 2 (Evaluation phase) |
Informed consent Block randomization – ReMiT-MS app-based intervention group and routine care group. |
Phase 2 will be conducted over six months. Baseline outcome measure assessment. Follow-up- 3rd and 6th month post-intervention. |
Primary outcome measure:
1) Multiple Sclerosis Quality of Life-54 questionnaires (MSQOL-54) [22]: The MSQOL-54 is a structured, self-reported questionnaire that will be completed by the individual with very little or no assistance. As a disease-specific measure, the MSQOL-54 reflects the overall impact of the disease and rehabilitation intervention on individuals with RRMS. The questionnaire has 54 items total, separated into 12 multi-item scales and two single items. MSQOL-54 scale scores are constructed by averaging items across scales and linearly transforming row scores to a scale of 0 -100 using the Likert method. Higher values show a higher quality of life.
Secondary outcomes measures:
2) Multiple Sclerosis Functional Composite (MSFC): The MSFC consists of three components that measure lower and upper extremity function and cognition in turn: the Timed 25-foot Walk [T25-FW]), the 9-hole Peg Test [9-HPT], and the Paced Auditory Serial Addition Test [PASAT]. The total MSFC score is calculated by averaging the Z- scores for each component. A change in cognition value of more than 0.5 standard deviation and a change in lower and upper extremity function values of greater than 20% is considered clinically meaningful [23].
3) Expanded disability status scale (EDSS): The EDSS is used to evaluate MS-related disabilities and monitor changes in disability levels over time. To calculate the EDSS score, eight functional systems (FSs) (plus “other”) are recognized [24]. The scale spans from 0 (normal neurological exam) to 10, representing the most serious outcome, which is death due to MS. It includes increments of 0.5–1.0, with higher scores indicating higher levels of disability.
4) Physical activity: International Physical Activity Questionnaires (IPAQ) [25]: Physical activity will be measured with the long version of the International Physical Activity Questionnaire (IPAQ). The questionnaire includes self- administered 27 items to measure physical activity participation during the previous seven-day period.
5) Modified Fatigue Impact Scale (MFIS) [26]: The MFIS is a 21-item questionnaire that measures physical, cognitive, and psychosocial fatigue, in individuals with MS. Each item is graded on a 5-point Likert scale. A total score is obtained by summing the scores for each item. Higher scores indicate a higher level of fatigue experienced over two weeks.
6) Berg Balance Scale (BBS) [27]: BBS is a broadly used clinical function test of an individual’s static and dynamic balance abilities in a clinical setting. A score of < 45 indicates an increased risk of falling. The psychometric properties of the scale have shown good intra- and inter-rater reliability in an MS patient [28].
7) Cost-effectiveness of mHealth app-based rehabilitation intervention: A bottom-up micro-costing approach will be adopted to estimate the costs associated with ReMiT-MS app-based exercise rehabilitation and the routine care group. A societal perspective will be considered for the costing, that comprehensively captures the costs incurred. Individuals in both groups will be instructed to maintain a diary; recording costs related to the management of RRMS during the study period from the date of enrollment to 6 months follow-up. Participants will be contacted once a month to share their diary entries. These will include out-of-pocket expenses associated with the management of RRMS. The overall costs will be categorized into the following sub-headings, namely Details of direct cost (expenditure incurred on specialist consultation, hospitalization, laboratory tests and other diagnostic procedures, medicines, and exercise therapy equipment such as exercise ball, weight cuffs, dumbbell, therabands, and non-elastic stretching belts). Indirect costs (absence from work; lost wages; time spent traveling between home and hospital and time spent in the hospital; cost of transport, accommodation, and food) will be collected. Additional costs related to persons accompanying the participants (under the above heads) will also be considered in the calculation. The cost incurred in purchasing and maintaining the mobile phones will be excluded as these are also used for other purposes in day-to-day routine life. Cost-effectiveness analysis: Incremental cost-effective ratios (ICERs) will be calculated for improvement in outcomes and quality-adjusted life-years (QALYs) gained. For each participant, ICER will be calculated by taking the per-individual mean cost difference (intervention minus control) and dividing it by the difference in their respective outcome measures.
Here ‘C’ and ‘E’ represent the cost and effectiveness in both groups of CEA.
8) Participant experience: At the second follow-up visit, only for the experimental group the qualitative interview will be conducted to explore participant’s experiences of using an app during a trial, as well as their willingness and ability to continue using the app in their everyday lives will be evaluated by subjective means through in-depth interviews. The participants will be selected using convenience sampling, and the sample size will be decided based on the principles of redundancy (information saturation).
Data analysis plan
IBM® SPSS Statistics 25.0 will be used to analyze the data. Descriptive statistics like number and proportion or mean/median and standard deviation/inter-quartile range will be calculated for the socio-demographic (age, sex, area of residence, education, occupation, and income), clinical (duration of disease, presence of comorbidity), rehabilitation (mode and duration of rehabilitation) and baseline outcome (QOL, Physical and Cognitive function) measures. The chi-square test would be applied for categorical variables, whereas the unpaired t-test or Mann-Whitney U test would be used for continuous variables to check the between-group difference in outcomes. Variables with p<0.1 in the univariate analysis will be included in the multivariable logistic analysis to assess the adjusted effect of mHealth app-based exercise rehabilitation intervention on improving the QOL and the effects will be presented as unadjusted and adjusted risk ratios. P<0.05 will be considered as statistically significant.
Ethical consideration and dissemination
All relevant ethical guidelines will be followed at each phase of the study. This study involves the education of individuals with RRMS about exercise rehabilitation interventions to manage impairments and disabilities. There will be strict adherence to the principles of the Declaration of Helsinki. The trial has been registered with the clinical trial registry of India (CTRI/2022/09/045266 on 06/09/2022). The ethical approval of the study has been taken from the institute ethics committee, PGIMER, Chandigarh, India (Ethical clearance number: (No: INT/IEC/2022/SPL-609; dated 25/5/2022), which is an independent body. All individuals will be given detailed facts about the study’s purpose and prerequisites. The significance of adhering to the exercise program and completing follow-up will be emphasized and a participant information sheet will be given. Informed written consent will be taken from the participants. Full autonomy will be provided to the participants for withdrawing from the study at any time without any adverse effects on their ongoing treatment at the neurology clinic. Study records will be maintained with a study identifier for each participant. The key to the identifier list will be accessible to the research team only during the study and will be recorded and retained by the principal investigator after the study is completed. The publications do not provide information about the identity of the patient. The confidentiality and anonymity of the participants will be ensured during the data collection and reporting of the results of the study. The study findings will be published in peer-reviewed journals. Participants will receive a lay summary of the findings if they have elected to receive study-level findings. The datasets used and/or analyzed during the current study will be made available by the corresponding author upon reasonable request and in agreement with the research collaboration.
Modification of the study: All study-related changes will be recorded, reported, and updated to the Institute Ethics Board, and the online trial registry (CTRI) will be updated accordingly. All the relevant changes will be informed to participants. Additional consent will be sought if necessary and documented.
The proposed study aims to develop and evaluate a user-centered and context-specific mHealth app for the rehabilitation of individuals with relapsing-remitting multiple sclerosis (RRMS). By leveraging the ubiquitous presence of smartphones, the mHealth app offers an opportunity for remote monitoring, scheduled interaction with experts, and instruction for exercise in a home environment.
The study addresses the challenges faced in routine care for individuals with MS, such as forgotten rehabilitation steps, limited access to local experts, and internal and external barriers to accessing care.
The study design consists of two phases. In Phase 1a, the mHealth app content will be developed based on a systematic review of the literature and an understanding of the health challenges and rehabilitation practices of individuals with RRMS. This phase will lay the foundation for the prototype of the mHealth app. Then, in Phase 1b, the usability of the app will be evaluated through a pilot study using the mHealth application usability questionnaire (MAUQ). Feedback from this phase will inform further modifications to the app’s content and workflow.
Phase 2 of the study will evaluate the clinical and cost-effectiveness of the ReMiT-MS app through a randomized controlled trial. The trial design follows a pragmatic approach, with individuals with RRMS randomized into two groups: the control group receiving routine care and the experimental group using the ReMiT-MS app-based exercise rehabilitation. Various outcome measures will be assessed, including the Multiple Sclerosis Quality of Life-54 questionnaire, Multiple Sclerosis Functional Composite, Expanded Disability Status Scale, physical activity levels, fatigue impact, balance, and cost-effectiveness. Comprehensive mHealth app-based exercise rehabilitation interventions described in the literature are in various stages of development, and validation studies for many of these apps are lacking. Given the low-cost potential of this form of intervention, it should be tested in a low-resource setting. The mHealth app is anticipated to be an effective service delivery model for individuals with MS for exercise rehabilitation measures, which may lead to improved quality of life, better adherence to home exercise programs, and maintaining or improving physical and cognitive function. It could therefore possibly lead to a reduction in the burden of the chronic progressive nature of this disease.
The proposed study has several strengths. First, it addresses the unmet needs in routine care for individuals with RRMS, particularly in resource-limited settings. By providing access to a user-friendly mHealth app, the study aims to overcome barriers related to forgotten rehabilitation steps and limited access to experts. Second, the study design follows a rigorous approach, including a mixed-methods development phase, a pilot phase to assess usability, and a randomized controlled trial to evaluate clinical and cost-effectiveness. Third, the study incorporates both objective and subjective outcome measures, allowing for a comprehensive assessment of the app’s impact on various aspects of individuals’ lives, including quality of life, physical and cognitive function, and cost.
However, the study also has limitations that need to be acknowledged. Firstly, the mHealth app is specifically designed for individuals with relapsing-remitting multiple sclerosis (RRMS). The app focuses on assessing and managing the symptoms associated with RRMS, but it cannot assess vital signs or gait disturbances. Secondly, the study relies on self-report measures for certain outcomes, which may introduce bias. Efforts will be made to minimize this by using validated questionnaires and maintaining blinding of assessors. Lastly, the study duration is limited to six months, which may not capture the long-term effects of the mHealth app intervention.
Trial status
Currently, we are recruiting patients for phase 2 of this study and the process was started on August 10, 2023.
The proposed research aims to address the challenges in routine care for individuals with RRMS by developing and evaluating a user-centered and context-specific mHealth app. The study design incorporates a mixed-methods approach for app development, usability assessment, and a randomized controlled trial to evaluate clinical and cost-effectiveness. The findings of this study may have implications for providing telerehabilitation and remote monitoring for individuals with RRMS in resource-limited settings and could inform the development of low-cost healthcare delivery models. Furthermore, the research may contribute to advancements in healthcare delivery not only in India but also in similar settings worldwide.
Declaration statements
Declaration of conflicting interests: There were no potential conflicts of interest disclosed by authors concerning the research, authorship, and/or publication of this article.
Contributorship: All authors made significant contributions to the concept design; or development of this research protocol. All authors contributed to the manuscript’s revision and edition and have given their final approval to the current version.
Guarantor: Corresponding author.
Financial disclosure: This research was conducted as an investigator-initiated trial and was not sponsored by any industry partners. The study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest related to the financial aspects of this study.
- Barten LJ, Allington DR, Procacci KA, Rivey MP. New approaches in the management of multiple sclerosis. Drug Des Devel Ther. 2010 Nov 24;4:343-66. doi: 10.2147/DDDT.S9331. PMID: 21151622; PMCID: PMC2998807.
- The Multiple Sclerosis International Federation Atlas of MS. Atlas of MS 3rd edition. Mult Scler Int Fed (MSIF), Sept 2020. 2020;(September):1–37.
- Singhal A, Bhatia R, Srivastava MV, Prasad K, Singh MB. Multiple sclerosis in India: An institutional study. Mult Scler Relat Disord. 2015 May;4(3):250-7. doi: 10.1016/j.msard.2015.03.002. Epub 2015 Mar 17. PMID: 26008942.
- Singhal B. Multiple sclerosis-Indian perspective. Neurol India. 2015 Nov-Dec;63(6):824-5. doi: 10.4103/0028-3886.170065. PMID: 26588610.
- Amatya B, Galea MP, Kesselring J, Khan F. Effectiveness of telerehabilitation interventions in persons with multiple sclerosis: A systematic review. Mult Scler Relat Disord. 2015 Jul;4(4):358-69. doi: 10.1016/j.msard.2015.06.011. Epub 2015 Jun 19. PMID: 26195057.
- Thirumalai M, Rimmer JH, Johnson G, Wilroy J, Young HJ, Mehta T, Lai B. TEAMS (Tele-Exercise and Multiple Sclerosis), a Tailored Telerehabilitation mHealth App: Participant-Centered Development and Usability Study. JMIR Mhealth Uhealth. 2018 May 24;6(5):e10181. doi: 10.2196/10181. PMID: 29798832; PMCID: PMC5992455.
- Khan F, Pallant JF, Brand C, Kilpatrick TJ. Effectiveness of rehabilitation intervention in persons with multiple sclerosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008 Nov;79(11):1230-5. doi: 10.1136/jnnp.2007.133777. Epub 2008 Jun 5. PMID: 18535027.
- Zulman DM, Jenchura EC, Cohen DM, Lewis ET, Houston TK, Asch SM. How Can eHealth Technology Address Challenges Related to Multimorbidity? Perspectives from Patients with Multiple Chronic Conditions. J Gen Intern Med. 2015 Aug;30(8):1063-70. doi: 10.1007/s11606-015-3222-9. Epub 2015 Feb 18. PMID: 25691239; PMCID: PMC4510242.
- Tacchino A, Pedullà L, Bonzano L, Vassallo C, Battaglia MA, Mancardi G, Bove M, Brichetto G. A New App for At-Home Cognitive Training: Description and Pilot Testing on Patients with Multiple Sclerosis. JMIR Mhealth Uhealth. 2015 Aug 31;3(3):e85. doi: 10.2196/mhealth.4269. PMID: 26323749; PMCID: PMC4704979.
- D'hooghe M, Van Gassen G, Kos D, Bouquiaux O, Cambron M, Decoo D, Lysandropoulos A, Van Wijmeersch B, Willekens B, Penner IK, Nagels G. Improving fatigue in multiple sclerosis by smartphone-supported energy management: The MS TeleCoach feasibility study. Mult Scler Relat Disord. 2018 May;22:90-96. doi: 10.1016/j.msard.2018.03.020. Epub 2018 Mar 27. PMID: 29649789.
- Babbage DR, van Kessel K, Drown J, Thomas S, Sezier A, Thomas P, Kersten P. MS Energize: Field trial of an app for self-management of fatigue for people with multiple sclerosis. Internet Interv. 2019 Nov 9;18:100291. doi: 10.1016/j.invent.2019.100291. PMID: 31890637; PMCID: PMC6926294.
- Giunti G, Mylonopoulou V, Romero OR. More stamina, a gamified mhealth solution for persons with multiple sclerosis: Research through design. J Med Internet Res. 2018;20(3).
- van Beek JJW, Lehnick D, Pastore-Wapp M, Wapp S, Kamm CP, Nef T, et al. Tablet app-based dexterity training in multiple sclerosis (TAD-MS): a randomized controlled trial. Disabil Rehabil Assist Technol [Internet]. 2022;0(0):1–11. Available from: https://doi.org/10.1080/17483107.2022.2131915
- Klimova B. Mobile applications used in multiple sclerosis [Internet]. Vol. 10995 LNCS, Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer International Publishing; 2018. 30–37 p. Available from: http://dx.doi.org/10.1007/978-3-319-97163-6_3
- Giunti G, Guisado Fernández E, Dorronzoro Zubiete E, Rivera Romero O. Supply and Demand in mHealth Apps for Persons With Multiple Sclerosis: Systematic Search in App Stores and Scoping Literature Review. JMIR Mhealth Uhealth. 2018 May 23;6(5):e10512. doi: 10.2196/10512. PMID: 29792295; PMCID: PMC5990860.
- Salimzadeh Z, Damanabi S, Kalankesh LR, Ferdousi R. Mobile Applications for Multiple Sclerosis: a Focus on Self-Management. Acta Inform Med. 2019 Mar;27(1):12-18. doi: 10.5455/aim.2019.27.12-18. PMID: 31213737; PMCID: PMC6511265.
- Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013 Jan 8;346:e7586. doi: 10.1136/bmj.e7586. PMID: 23303884; PMCID: PMC3541470.
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 Feb;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2. Epub 2017 Dec 21. PMID: 29275977.
- Zhou L, Bao J, Setiawan IMA, Saptono A, Parmanto B. The mHealth App Usability Questionnaire (MAUQ): Development and Validation Study. JMIR Mhealth Uhealth. 2019 Apr 11;7(4):e11500. doi: 10.2196/11500. PMID: 30973342; PMCID: PMC6482399.
- Carter AM, Daley AJ, Kesterton SW, Woodroofe NM, Saxton JM, Sharrack B. Pragmatic exercise intervention in people with mild to moderate multiple sclerosis: a randomised controlled feasibility study. Contemp Clin Trials. 2013 Jul;35(2):40-7. doi: 10.1016/j.cct.2013.04.003. Epub 2013 Apr 21. PMID: 23612222.
- Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E, Giesser B, Halper J, Karpatkin H, Keller J, Ng AV, Pilutti LA, Rohrig A, Van Asch P, Zackowski K, Motl RW. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler. 2020 Oct;26(12):1459-1469. doi: 10.1177/1352458520915629. Epub 2020 Apr 23. PMID: 32323606; PMCID: PMC7575303.
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995 Jun;4(3):187-206. doi: 10.1007/BF02260859. PMID: 7613530.
- Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999 Aug;5(4):244-50. doi: 10.1177/135245859900500409. PMID: 10467383.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444-52. doi: 10.1212/wnl.33.11.1444. PMID: 6685237.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003 Aug;35(8):1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB. PMID: 12900694.
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994 Jan;18 Suppl 1:S79-83. doi: 10.1093/clinids/18.supplement_1.s79. PMID: 8148458.
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992 Jul-Aug;83 Suppl 2:S7-11. PMID: 1468055.
- Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006 Jun 30;28(12):789-95. doi: 10.1080/09638280500404289. PMID: 16754576.