Research Article
Factors affecting muscle strength in cancer patients receiving chemotherapy

Jiro Nakano1*, Shun Ishii2, Takuya Fukushima2,3, Ayumi Natsuzako2, Junya Sakamoto1 and Minoru Okita3
1Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, Japan
2Department of Rehabilitation, Nagasaki University Hospital, Japan
3Department of Locomotive Rehabilitation Science, Nagasaki University Graduate School of Biomedical Sciences, Japan
*Address for Correspondence: Dr. Jiro Nakano, Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki city, Nagasaki 852 8520, Japan, Tel/Fax: +81 95 819 7919; Email: [email protected]
Dates: Submitted: 12 June 2017; Approved: 07 July 2017; Published: 10 July 2017
How to cite this article: Nakano J, Ishii S, Fukushima T, Natsuzako A, Sakamoto F, et al. Factors affecting muscle strength in cancer patients receiving chemotherapy. J Nov Physiother Rehabil. 2017; 1: 056-066.
DOI: 10.29328/journal.jnpr.1001008
Copyright License: © 2017 Nakano J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cancer; Chemotherapy; Solid tumor; Hematological malignancy; Muscle strength; Hemoglobin; Fatigue
ABSTRACT
This study aimed to investigate the relationship between muscle weakness and cancer-related symptoms in patients undergoing chemotherapy for hematological malignancies and solid tumors. We recruited hospitalized patients older than 20 years who were receiving chemotherapy. Patients were divided into a solid tumor (n=74) and hematological malignancy (n=80) group. Age, body mass index (BMI), strength and thickness of the quadriceps femoris muscle, serum albumin and C-reactive protein levels, blood hemoglobin concentration, fatigue, psychological distress and pain, and duration of hospitalization were assessed. Eight physical symptoms (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea) were also evaluated. Correlation and multiple regression analyses were conducted to identify factors affecting muscle strength in each group. Muscle strength was associated with fatigue in the solid tumor group and with age, BMI, muscle thickness, albumin and hemoglobin in the hematological malignancy group. Therefore, factors contributing to muscle strength might differ between patients with solid tumors and those with hematological malignancies. In particular, fatigue was an important factor in patients with solid tumors, while anemia was an important factor in patients with hematological malignancies. We therefore suggest that different treatments for muscle weakness might be considered for patients with these cancer types.
INTRODUCTION
Muscle weakness is an important adverse effect in cancer patients that negatively affects their ability to perform daily activities and quality of life [1]. This weakness can largely explained by cancer-related muscle wasting, cachexia, and disuse muscle atrophy or sarcopenia [2,3]. In particular, cachexia was believed to result from activation of the acute phase response whereby pro-inflammatory cytokines are upregulated [4]. This in turn leads to altered protein, fat, and carbohydrate metabolism, resulting in body weight reduction as well as changes in body composition [5]. The incidence of cachexia among cancer patients is very high and estimated to affect 50-80% of patients, although this rate varies by tumor type [6].
Muscle weakness in cancer patients also influenced by other factors such as physical symptoms and psychological and environmental problems. For example, muscle weakness in cancer patients is strongly associated with cancer-related fatigue [7]. Fatigue in patients with cancer is also frequently associated with various forms of psychosocial distress (e.g., clinical depression, anxiety, and coping with chronic illness), and exacerbating symptoms (e.g., anemia, pain, dyspnea, insomnia, nausea) [8], as well as side effects of chemotherapy [9]. Moreover, anxiety and depression induced by cancer-related factors such as fatigue and diagnosis might reduce patients’ physical ability, leading to the progression of disuse muscle atrophy [10]. Limitation of activities during hospitalization for infection prophylaxis or medical treatment such as intravenous feeding may also cause disuse muscle atrophy [11].
When factors contributing to muscle weakness are compared across different cancer types, interesting differences can be seen between solid tumors and hematological malignancies. For example, anemia is present in 54% of cancer patients, and increases to 75% in patients undergoing chemotherapy [12]. However, the prevalence of anemia in patients with hematological malignancies is double that in patients with solid tumors [9,13]. Since anemia is strongly associated with disability, poorer physical performance, and muscle weakness in the community-dwelling elderly [14], anemia can also possibly affect muscle strength in patients with cancer [2]. Moreover, the prevalence of malnutrition in patients with solid tumors is higher than in patients with hematological malignancies [15]. Patients with solid tumors undergoing chemotherapy also frequently experience appetite loss, nausea, and vomiting. These symptoms lead to induction of fatigue [8], as well as malnutrition that further promotes cachexia and muscle weakness [16].
Furthermore, muscle weakness possibly contributes not only to loss of muscle mass but also to muscle activation in cancer patients receiving chemotherapy. Oxidative stress mediated by reactive oxygen species or by chemotherapeutic agents leads to muscle contractile dysfunction and fatigue [17,18]. In fact, muscle strength of cancer patients with severe fatigue was reported to be weaker than that of patients with light fatigue, although muscle volume was the same [19]. The symptoms and loss of muscle activation might be different between solid tumors and hematological malignancies [20].
From the above, we hypothesized that the main factors associated with muscle weakness differ between solid tumors and hematological malignancies. In order to optimize cancer treatments, these differences need to be clarified, and we believe that this will be particularly important for rehabilitative purposes. Therefore, this study aimed to investigate factors affecting muscle weakness in cancer patients undergoing chemotherapy and to examine whether these factors differ between patients with solid tumors and hematological malignancies.
MATERIALS AND METHODS
Setting and participants
This cross-sectional study was conducted at Nagasaki University Hospital, from January 2014 to March 2016. This study was approved by the hospital ethics committee before any patients were enrolled (approval number 12092419). The patients were given a written letter informing them of the aims and objectives of the study, and written consent was obtained from every participant. Patients older than 20 years, who had been diagnosed with cancer, and hospitalized in order to receive cancer treatment were eligible. In order to minimize bias due to differences among solid tumor types, only patients with lung, kidney, uterus, esophagus, liver, large bowel, stomach, breast and pancreatic cancers were eligible. Patients were also excluded if 1) they had undergone tumorectomy prior to this study, 2) had received radiotherapy, 3) had dementia or disturbed consciousness, 4) had been recommended bedrest (Eastern Cooperative Oncology Group [ECOG] performance status=4), 5) had received bone marrow transplantation, and 5) did not agree to participate in this study. General and clinical information about the patients such as age, sex, height, weight, body mass index (BMI), diagnosis, ECOG performance status, and date of hospital admission and initiation of chemotherapy were recorded. Patients were categorized into either the solid tumor or hematological malignancy group based on their diagnosed cancer type. Patients who were diagnosed with both cancer types were excluded.
Muscle strength
The strength of the quadriceps femoris muscle was assessed. Muscle strength was quantified using maximum voluntary isometric contraction for knee joint extension, in accordance with a previous study [21]. Patients were seated at the corner of a mat table, with their knees bent at approximately 90 degrees flexion. The mat table had a height such that when the subjects sat on it; both their feet just reached the floor. A portable muscle force dynamometer (µ-tas MF-01, ANIMA Corp., Tokyo, Japan) was placed against the anterior side of the patients’ legs, just proximal to the malleoli. The dynamometer stabilizing belt passed around the legs of the bed. Patients were then asked to continue trying to straighten their knees with maximum effort until the tester asked them to stop for about 5 seconds. During the measurements, the examiner provided verbal encouragement and held both sides of the sensor, to maintain the direction of the sensor, so that the surface of the sensor was kept relative to the direction of the movement. The measurement was conducted three times for one leg, with an interval of 30 seconds or more between measurements. The measurements were obtained for both the right and left legs, and data from the stronger leg were used for analysis.
Muscle thickness
Muscle mass was quantified by measuring the thickness of the vastus intermedius plus rectus femoris muscles, in accordance with a previous study [22]. Muscle thickness was determined using an ultrasound device (SeeMoreTM, Interson Corp., CA, USA), with the patient in a supine position and his or her legs lying flat and relaxed in extension. On the same leg from which muscle strength data were used for analysis, a straight line was drawn between the anterior superior iliac spine and upper margin of the patella. Thickness measurements were then taken at a level two thirds of the distance down this line.
Blood chemistry
Serum albumin, serum C-reactive protein (CRP), and corpuscular hemoglobin concentrations were measured with a standard clinical analyzer for the blood samples of all patients. Serum albumin level was chosen as it is a well-known marker of nutritional status. Serum CRP level, which is a sensitive marker of inflammation, is known to be associated with cancer cachexia [23]. Lastly, hemoglobin is an important clinical parameter for assessing anemia [24]. It has also been reported that all blood biochemical parameters are associated with muscle strength or mass [25,26].
Cancer-related fatigue
Fatigue was assessed using the Cancer Fatigue Scale (CFS), a valid and brief self-rating scale for assessing cancer-related fatigue that was designed specifically to reflect the nature of the fatigue [27]. The scale consists of 15 items that are each rated on a scale of 1 (“not at all”) to 5 (“very much”), and patients are asked to circle the number that describes their current state. The scale provides a total score ranging from 0 to 60, with a total score of 23 points or more indicating the presence of fatigue symptoms [28]. Additionally, to classify cancer fatigue further and perform subgroup analyses, the tool can be divided into physical, affective, and cognitive subscales.
Pain
Pain was recorded using the numerical rating scale (NRS) score (0: no pain, 10: worst imaginable pain). It is widely known that cancer patients experience various forms of pain including somatic, visceral, neuropathic and mixed [29]. Moreover, pain can be classified as acute pain or chronic pain. In this study, pain was evaluated regardless of pain type or source when measuring muscle strength.
Psychological distress
The summed total score of the Hospital Anxiety and Depression Scale (HADS) was used to assess psychological distress [30]. This is a widely used and validated questionnaire to measure psychological morbidity in cancer patients [30]. It consists of a 7-item anxiety subscale and a 7-item depression subscale. Each item is rated on a scale of 0 (best status) to 3 (worst status). Possible scores for the anxiety and depression subscales each range from 0 to 21 points. Eleven points or more on either subscale are considered to be a significant ‘case’ of psychological morbidity [31].
STATISTICAL ANALYSIS
Data analysis was carried out with SPSS for Windows version 23 (IBM-SPSS Inc., Chicago, USA). Values were expressed as mean±deviation. Where appropriate, group comparisons were carried out using contingency table analysis (chi-square analysis) and the Mann-Whitney U test. Correlation coefficients between muscle strength and other parameters were calculated using Pearson’s test. Additionally, a multiple linear regression analysis was performed separately for each group. Muscle strength was defined as the dependent variable, and independent variables were defined based on correlation coefficients between the assessed factors and muscle strength: parameters which showed significant relationship with muscle strength (p<0.05 in analysis with data of all patients) were selected. To avoid multicollinearity, only one variable was selected if there was a significant correlation (r>0.7) between two independent variables. The model fit was assessed using the appropriate residual and goodness-of-fit statistic. The threshold for significance was set at 0.05 for all statistical analyses.
RESULTS
Subjects’ characteristics
Results from 163 cancer patients were used for analysis. The clinical characteristics of patients are shown in table 1. Based on clinical assessment, 74 patients were included in the solid tumor group and 89 in the hematological malignancy group. The solid tumor group included a large proportion of lung (28.4%) and kidney (17.6%) tumors. In the hematological malignancy group, malignant lymphoma had the highest prevalence. There were no significance differences in age, gender, BMI, ECOG performance status, number of days after hospital admission or initiation of chemotherapy between the solid tumor and hematological malignancy groups. Although the numbers of days after hospital admission and chemotherapy initiation showed variation, the average of the number of days after hospital admission was approximately 14 in both the solid tumor and hematological malignancy groups and there were no significant differences between groups.
| Table 1: Clinical characteristics of cancer patients. | ||||
| Total | Solid tumor | Hematological malignancy | P | |
| (n=163) | (n=74) | (n=89) | ||
| Age (years) | 67.4±12.5 | 68.1±12.8 | 65.8±12.1 | 0.081 |
| Sex | 0.586 | |||
| Male, n (%) | 90 (53.9) | 41 (56.6) | 49 (55.6) | |
| Female, n (%) | 73 (46.1) | 33 (43.4) | 40 (44.4) | |
| BMI (Kg/m2) | 21.1±3.8 | 21.5±4.2 | 20.8±3.4 | 0.169 |
| Type of cancer, n (%) | ||||
| Lung | 21 (28.4) | |||
| Kidney | 13 (17.6) | |||
| Uterus | 10 (13.5) | |||
| Esophagus | 10 (13.5) | |||
| Liver | 5 (6.8) | |||
| Large bowel | 7 (9.5) | |||
| Stomach | 5 (6.8) | |||
| Pancreas | 3 (4.1) | |||
| Malignant lymphoma | 41 (46.1) | |||
| Myelogenous leukemia | 28 (31.5) | |||
| Multiple myeloma, | 9 (10.1) | |||
| Myelodysplastic syndrome | 6 (6.7) | |||
| Lymphocytic leukemia | 5 (5.6) | |||
| Performance status, n (%) | 0.575 | |||
| Score 0 | 1 (0.6) | 1 (1.4) | 0 (0) | |
| Score 1 | 54 (33.1) | 24 (32.4) | 30 (33.7) | |
| Score 2 | 63 (38.7) | 30 (40.5) | 33 (37.1) | |
| Score 3 | 45 (27.6) | 19 (25.7) | 26 (29.2) | |
| Days after hospitalization | 14.2±16.3 | 14.0±11.6 | 14.4±19.3 | 0.071 |
| Days after chemotherapy | 5.8±8.7 | 5.6±8.0 | 5.9±9.3 | 0.831 |
| BMI: Body Mass Index. Days after hospitalization: number of days after hospital admission. Days after chemotherapy: number of days after initiation of chemotherapy. P: p-values, chi-square analysis or Mann-Whitney U test for comparison of solid tumor and hematological malignancy groups. | ||||
Comparison of outcome measures
All muscle strength and thickness measurements are shown in table 2. No significant differences in muscle strength or thickness were found between the solid tumor and hematological malignancy groups. Regarding blood chemistry, both the solid tumor and hematological malignancy groups showed abnormal values compared with the reference ranges; namely, serum albumin and hemoglobin levels were low, while serum CRP levels were high.
| Table 2: Summary of measurements. | ||||
| Parameter | Total | Solid tumor | Hematological malignancy | P |
| (n=163) | (n=74) | (n=89) | ||
| Muscle strength (Kg) | 22.1±11.4 | 21.1±10.8 | 23.0±11.8 | 0.202 |
| Muscle thickness (mm) | 14.9±5.0 | 14.9±5.4 | 14.8±4.8 | 0.475 |
| Blood chemistry | ||||
| Albumin (g/dl) | 3.2±0.7 | 3.1±0.7 | 3.4±0.6 | 0.006 |
| CRP (mg/dl) | 2.3±4.1 | 3.1±4.5 | 1.6±3.7 | 0.023 |
| Hemoglobin (g/dl) | 9.8±2.0 | 10.3±2.1 | 9.6±2.0 | 0.013 |
| CFS | ||||
| Total | 21.1±9.4 | 21.9±10.4 | 20.4±8.4 | 0.315 |
| Physical | 8.3±5.4 | 8.7±5.9 | 8.0±5.1 | 0.450 |
| Affective | 8.4±5.5 | 8.3±3.0 | 8.4±3.4 | 0.736 |
| Cognitive | 4.4±3.4 | 4.9±3.7 | 3.9±3.1 | 0.065 |
| Pain (NRS) | 2.8±2.9 | 3.6±3.5 | 2.3±2.5 | 0.049 |
| HADS | ||||
| Total | 13.3±6.2 | 13.1±6.7 | 13.5±5.8 | 0.712 |
| Anxiety | 6.1±3.5 | 6.0±3.5 | 6.2±3.4 | 0.790 |
| Depression | 7.1±3.7 | 7.1±3.9 | 7.2±3.5 | 0.916 |
| Reference range of blood chemistry: albumin, 3.5-5.0 g/dl; Hemoglobin, male, 13.0-16.5 g/dl; female, 11.5-14.7 g/dl; CRP, <0.25 mg/dl. CRP: C-reactive protein, CFS: Cancer Fatigue Scale. HADS: Hospital Anxiety and Depression Scale, NRS: Numerical Rating Scale. P: p-values, Mann-Whitney U test for comparison of solid tumor and hematological malignancy groups. | ||||
In terms of between-group differences, the solid tumor group showed significantly lower serum albumin levels (p=0.006), and significantly higher serum CRP (p=0.023) and hemoglobin levels (p=0.013) than the hematological malignancy group. On the other hand, mean CFS scores did not significantly differ between the two groups. There were also no significant between-group differences when CFS subscales were analyzed. In particular, “fatigue” morbidity, as defined by a CFS score ≥23 points [28], was 32.4% (24 patients) in the solid tumor group and 37.1% (33 patients) in the hematological malignancy group; these differences were not statistically significant (p=0.649; chi-square analysis). The solid tumor group showed a higher pain score than the hematological malignancy group, but the difference was small (p=0.049). The proportion of patients with pain in the solid tumor and hematological malignancy group was 27.0% (20 patients) and 30.3% (27 patients), respectively: there were no statistically significant differences between the two groups (p=0.770; chi-square analysis). HADS total and subclass (anxiety and depression) scores did not differ significantly between the two groups. Major depressive disorder morbidities (total ≥20 points) [32] were 12.1% (9 patients) in the solid tumor group and 14.6% (13 patients) in the hematological malignancy group (p=0.821; chi-square analysis).
Correlation between muscle strength and other parameters
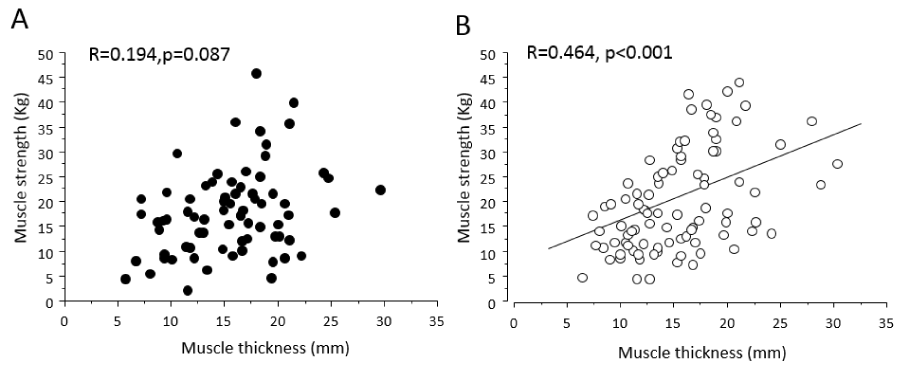
When the data from all patients were analyzed together, factors that were significantly correlated with muscle strength were age, BMI, muscle thickness, serum albumin, hemoglobin, CFS, number of days after hospital admission and chemotherapy (Table 3). When correlation analyses were performed in the solid tumor and hematological malignancy groups separately, several differences between the two groups were observed. Muscle thickness (r=0.464) was positively correlated with muscle strength in the hematological malignancy group only (Figure 1). In the solid tumor group, only CFS scores (r=-0.395) were negatively correlated with muscle strength. In contrast, significant correlations between muscle strength and age (r=-0.294) and albumin (r=0.218) were observed in the hematological malignancy group. The only factors significantly correlated with muscle strength in both groups were BMI and hemoglobin. Pain, HADS total scores, and number of days after hospital admission and chemotherapy were not associated with muscle strength in either group.
| Table 3: Correlation between muscle strength and other parameters. | ||||||||
| Total | Solid tumor | Hematological malignancy | ||||||
| Parameter | R | P | R | P | R | P | ||
| Age | ±0.217 | 0.004 | ±0.055 | 0.637 | ±0.294 | 0.003 | ||
| BMI | 0.308 | <0.001 | 0.311 | 0.005 | 0.335 | 0.007 | ||
| Muscle thickness | 0.342 | <0.001 | 0.194 | 0.087 | 0.464 | <0.001 | ||
| Blood chemistry | ||||||||
| Albumin | 0.217 | 0.005 | 0.188 | 0.129 | 0.218 | 0.033 | ||
| CRP | ±0.150 | 0.056 | ±0.195 | 0.103 | ±0.085 | 0.424 | ||
| Hemoglobin | 0.248 | <0.001 | 0.307 | 0.006 | 0.240 | 0.018 | ||
| CFS (Total) | ±0.282 | <0.001 | ±0.395 | 0.001 | ±0.163 | 0.141 | ||
| Pain (NRS) | ±0.122 | 0.284 | ±0.305 | 0.101 | ±0.053 | 0.721 | ||
| HADS (Total) | ±0.059 | 0.493 | ±0.094 | 0.476 | ±0.041 | 0.725 | ||
| Days after hospitalization | ±0.034 | 0.659 | ±0.194 | 0.916 | ±0.036 | 0.724 | ||
| Days after chemotherapy | ±0.087 | 0.250 | ±0.025 | 0.830 | ±0.129 | ±0.209 | ||
| BMI: Body Mass Index. CRP: C-reactive protein. CFS: Cancer Fatigue Scale. HADS: Hospital Anxiety and Depression Scale. Days after hospitalization: number of days after hospital admission. Days after chemotherapy: number of days after initiation of chemotherapy. R: Pearson’s correlation coefficient. P: p-values. | ||||||||
Figure 1: Relationships between muscle strength and muscle thickness of the quadriceps femoris muscle in the solid tumor (A) and the hematological malignancy groups (B).
In the multiple regression analysis, age, BMI, muscle thickness, serum albumin, hemoglobin, and CFS score were selected as independent variables. The only factor significantly associated with muscle strength was CFS score (β=-0.355, p=0.018) in the solid tumor group. Age, BMI, muscle thickness, serum albumin and hemoglobin were not significantly associated with muscle strength (Table 4). In the hematological malignancy group, age, BMI, muscle thickness, and hemoglobin were significantly associated with muscle strength, with a strong association between muscle strength and age (β=0.257, p=0.014) and hemoglobin (β=0.240, p=0.012).
| Table 4: Multiple regression analysis for factors affecting muscle strength. | ||||||||
| Total | Solid tumor | Hematological malignancy | ||||||
| Parameter | β | P | β | P | β | P | ||
| Age | -0.167 | 0.045 | -0.046 | 0.739 | -0.257 | 0.014 | ||
| BMI | 0.158 | 0.126 | 0.068 | 0.710 | 0.240 | 0.047 | ||
| Muscle thickness | 0.152 | 0.132 | 0.003 | 0.988 | 0.236 | 0.050 | ||
| Albumin | 0.015 | 0.857 | 0.021 | 0.894 | 0.043 | 0.657 | ||
| Hemoglobin | 0.210 | 0.011 | 0.147 | 0.356 | 0.240 | 0.012 | ||
| CFS | -0.207 | 0.013 | -0.355 | 0.018 | -0.119 | 0.237 | ||
| R2 | 0.245 | 0.212 | 0.375 | |||||
| CFS: Cancer Fatigue Scale. β; standardised partial regression coefficient.P: p-values | ||||||||
DISCUSSION
This study investigated and compared factors associated with muscle weakness in patients with solid tumors and hematological malignancies receiving chemotherapy. Although the characteristics of skeletal muscle weakness in cancer patients have been described previously, this study was the first to investigate muscle strength in adult patients with hematological malignancy receiving chemotherapy.
Significant between-group differences were detected in serum albumin, CRP, hemoglobin, and pain. The malnutrition was significantly more severe in patients with a solid tumor. This is consistent with a previous report that found that the prevalence of malnutrition is higher in cancer patients with solid tumors than in patients with hematological malignancies [15]. Additionally, patients in the solid tumor group showed high serum levels of the inflammatory marker CRP. A previous study has reported that systemic inflammation, CRP levels, is associated with malnutrition in cancer patients [33]. Since malnutrition and systemic inflammation are the most important factors in the induction of cachexia [4], muscle weakness by cachexia would theoretically be expected to be severe in the solid tumor group. However, there was no difference in the strength and thickness of the quadriceps femoris muscle between patients in the solid tumor and hematological malignancy groups. This contradiction suggests that muscle strength in patients of both groups was affected by factors other than cachexia. In this cross-sectional study, it was difficult to detect between-group differences in factors affecting muscle strength by comparing mean values for the measured parameters. Therefore, correlation and multiple regression analyses were then conducted in each group.
The multiple regression analysis demonstrated that only CFS score was a significant independent variable in the solid tumor group. It has been also reported that muscle weakness in patients with cancer is strongly related to fatigue [7]. Therefore, we postulate that the influence of age and muscle mass, and thereby cachexia, on muscle strength in solid tumor group patients was masked by their strong fatigue. In contrast, correlations between muscle strength and age, BMI, and muscle thickness were shown in the hematological malignancy group, as described in a previous study on healthy participants [34,35]. Given the lack of association between muscle strength with serum albumin and CRP levels, it can be deduced that the influence of cachexia on muscle strength might be small [23,24]. Thus, if patients in the hematological malignancy group displayed muscle weakness, this weakness must be based on sarcopenia and/or disuse muscle atrophy [36]. However, a significant correlation between hemoglobin concentration and muscle strength was detected; hemoglobin was additionally defined as a factor affecting muscle strength in a multiple regression analysis. Decline of hemoglobin concentration is one cause of anemia [24], and anemia is strongly associated with muscle weakness [2,14]. In animal experiments, it has been shown that a decline in hemoglobin concentration and anemia may induce negative changes in mitochondria and biosynthetic enzymes in muscle cells [37,38]. Recently, mitochondrial dysfunction was also identified in an animal model as one cause of cancer-related muscle weakness [39]. Thus, anemia may directly affect muscle strength in cancer patients.
Anemia in cancer patients frequently develops not only as a direct symptom of cancer, but also as an adverse effect of chemotherapy [12,40,41]. In this study, patients in both the solid tumor and the hematological malignancy group received chemotherapy. However, hemoglobin concentration was identified as a factor affecting muscle strength only in the hematological malignancy group. Additionally, hemoglobin levels in the hematological malignancy group were significantly lower than in the solid tumor group. Low hemoglobin levels might therefore be an important factor affecting muscle strength in patients with hematological malignancy.
Some questions remain regarding the results of this study. For example, despite the fact that hemoglobin concentration among participants in the solid tumor group was lower than that in healthy individuals [28], this biochemical marker was not identified as a significant factor influencing muscle strength. Similarly, fatigue in the hematological malignancy group was not found to be a factor contributing to muscle strength. These unexplained results suggest the presence of other important factors that were not analyzed in this study. Additionally, several limitations inherent to this study should be considered.
First, the measurement of muscle strength and knee extension was not conducted according to a conventional method: a chair with a back and a fixed belt for the trunk were not used in this study. It was also not clear whether the cancer patients who were mentally weak made the greatest efforts. Other limitations were as follows: The study had a small sample size. The solid tumor group consisted of patients with tumors in various organs. The conditions of chemotherapy such as frequency, dose, and duration were not investigated in detail. Although the period from induction of cancer (diagnosis) to measurement and staging of cancer should be considered, these data were not included in analysis. The physical activity of patients diagnosed with cancer must have decreased, which would have contributed to muscle weakness [42].
CONCLUSION
We found that causes of declining muscle strength differ between solid tumor and hematological malignancy patients undergoing chemotherapy. Although fatigue appeared to be the most important factor affecting muscle weakness in patients with solid tumors, low hemoglobin level was an important factor in patients with hematological malignancies. In the clinic, gaining muscle hypertrophy through intensive resistance training is often difficult for cancer patients undergoing chemotherapy because of fatigue, malnutrition, and anemia. Based on the results of this study, low-intensity exercise could be recommended for a part of patients with solid tumors who have severe fatigue, as low-intensity exercise is known to reduce fatigue [43,44]. This could improve muscle weakness even if muscle mass is not changed. On the other hand, it has been reported in animal experiments that aerobic exercise is effective in improving low hemoglobin levels and anemia [45]. However, effects and safety of aerobic exercise with regard to anemia have yet to be confirmed in patients with hematological malignancies. Future research should investigate the most effective exercises for improving muscle weakness in cancer patients undergoing chemotherapy.
FUNDING
This work is supported by JSPS KAKENHI Grant Number JP26282156.
REFERENCES
- Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin Nutr. 2012; 31: 499-505. Ref.: https://goo.gl/rjsncd
- Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Muscle wasting in cancer and ageing: cachexia versus sarcopenia. Adv Gerontol. 2006; 18: 39-54. Ref.: https://goo.gl/fX8mEc
- Toth MJ, Callahan DM, Miller MS, Tourville TW, Hackett SB, et al. Skeletal muscle fiber size and fiber type distribution in human cancer: Effects of weight loss and relationship to physical function. Clin Nutr. 2016; 35: 1359-1365. Ref.: https://goo.gl/Xbhqw7
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009; 89: 381-410. Ref.: https://goo.gl/JgiKgB
- Bongaerts GP, van Halteren HK, Verhagen CA, Wagener DJ. Cancer cachexia demonstrates the energetic impact of gluconeogenesis in human metabolism. Med Hypotheses. 2006; 67: 1213-1222. Ref.: https://goo.gl/46H3YQ
- Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014; 14: 754-762. Ref.: https://goo.gl/2QJWF9
- Kilgour RD, Vigano A, Trutschnigg B, Hornby L, Lucar E, et al. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2011; 1: 177-185. Ref.: https://goo.gl/Gzo5zX
- Yennurajalingam S, Palmer JL, Zhang T, Poulter V, Bruera E. Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer. 2008; 16: 1125-1130. Ref.: https://goo.gl/bVUaXg
- Berglund G, Bolund C, Fornander T, Rutqvist LE, Sjoden PO. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Eur J Cancer. 1991; 27: 1075-1081. Ref.: https://goo.gl/4Y1Jrn
- Lee D, Hwang JH, Chu I, Chang HJ, Shim YH, et al. Analysis of factors related to arm weakness in patients with breast cancer-related lymphedema. Support Care Cancer. 2015; 23: 2297-2304. Ref.: https://goo.gl/tJmRuU
- Kawahara K, Suzuki T, Yasaka T, Nagata H, Okamoto Y, et al. Evaluation of the site specificity of acute disuse muscle atrophy developed during a relatively short period in critically ill patients according to the activities of daily living level: A prospective observational study. Aust Crit Care. 2017; 30: 29-36. Ref.: https://goo.gl/uorwVX
- Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004; 40: 2293-2306. Ref.: https://goo.gl/KVMrT8
- Birgegard G, Gascon P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J Haematol. 2006; 77: 378-386. Ref.: https://goo.gl/HxtQmR
- Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004; 52: 719-724. Ref.: https://goo.gl/bjvLnR
- Tah PC, Nik Shanita S, Poh BK. Nutritional status among pediatric cancer patients: a comparison between hematological malignancies and solid tumors. J Spec Pediatr Nurs. 2012; 17: 301-311. Ref.: https://goo.gl/ZdaJU7
- Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition. 2005; 21: 977-985. Ref.: https://goo.gl/eCS5bE
- Gilliam LA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011; 15: 2543-2563. Ref.: https://goo.gl/oofZG9
- Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015; 5: 356-377. Ref.: https://goo.gl/BVEjcz
- Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005; 103: 377-382. Ref.: https://goo.gl/rXCwn4
- Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011; 34: 292-296. Ref.: https://goo.gl/Adq1Qp
- Katoh M, Kaneko Y. An Investigation into Reliability of Knee Extension Muscle Strength Measurements, and into the Relationship between Muscle Strength and Means of Independent Mobility in the Ward: Examinations of Patients Who Underwent Femoral Neck Fracture Surgery. JPhys Ther Sci. 2014; 26: 15-19. Ref.: https://goo.gl/VogqKn
- Tillquist M, Kutsogiannis DJ, Wischmeyer PE, Kummerlen C, Leung R, et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr. 2013; 38: 886-890. Ref.: https://goo.gl/zG3Wyu
- Wu YY, Chao TY, Liu HY, Huang TC, Chen JH, et al. The correlation between a chronic inflammatory marker Tartrate-resistant acid phosphatase 5a with cancer cachexia. J BUON. 2015; 20: 325-331. Ref.: https://goo.gl/RrHHvk
- Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006; 107: 1747-1750. Ref.: https://goo.gl/FWrs1z
- Kuzuya M, Izawa S, Enoki H, Okada K, Iguchi A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin Nutr. 2007; 26: 84-90. Ref.: https://goo.gl/AFzBEu
- Pires Corona L, Drumond Andrade FC, de Oliveira Duarte YA, Lebrao ML. The Relationship between Anemia, Hemoglobin Concentration and Frailty in Brazilian Older Adults. J Nutr Health Aging. 2015; 19: 935-940. Ref.: https://goo.gl/qNTSaz
- Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000; 19: 5-14. Ref.: https://goo.gl/6dww2M
- Kroz M, Zerm R, Reif M, HB VONL, Schad F, et al. Validation of the German version of the Cancer Fatigue Scale (CFS-D). Eur J Cancer Care (Engl). 2008; 17: 33-41. Ref.: https://goo.gl/YVXvZ4
- Lasheen W, Walsh D, Sarhill N, Davis M. Intermittent cancer pain: clinical importance and an updated cancer pain classification. Am J Hosp Palliat Care. 2010; 27: 182-186. Ref.: https://goo.gl/3kyE71
- Vodermaier A, Millman RD. Accuracy of the Hospital Anxiety and Depression Scale as a screening tool in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2011; 19: 1899-1908. Ref.: https://goo.gl/tvges4
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67: 361-370. Ref.: https://goo.gl/5kkgKr
- Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998; 28: 333-338. Ref.: https://goo.gl/gQ7CGX
- Tan CS, Read JA, Phan VH, Beale PJ, Peat JK, et al. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer. 2014; 23: 385-391. Ref.: https://goo.gl/euXKgM
- Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983; 338: 37-49. Ref.: https://goo.gl/pEZfPK
- Hayashida I, Tanimoto Y, Takahashi Y, Kusabiraki T, Tamaki J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS One. 2014; 9: e111810. Ref.: https://goo.gl/VZfrKM
- Ryan AM, Power DG, Daly L, Cushen SJ, Ni Bhuachalla E, et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016; 75: 199-211. Ref.: https://goo.gl/QWjuRT
- Tanne Z, Coleman R, Nahir M, Shomrat D, Finberg JP, et al. Ultrastructural and cytochemical changes in the heart of iron-deficient rats. Biochem Pharmacol. 1994; 47: 1759-1766. Ref.: https://goo.gl/kWfXkY
- McNabney LA, Essig DA. 5'-Aminolevulinate synthase activity is decreased in skeletal muscle of anemic rats. Am J Physiol. 1992; 263: C429-435. Ref.: https://goo.gl/nQU3bP
- Antunes D, Padrao AI, Maciel E, Santinha D, Oliveira P, et al. Molecular insights into mitochondrial dysfunction in cancer-related muscle wasting. Biochim Biophys Acta. 2014; 1841: 896-905. Ref.: https://goo.gl/AF9wg5
- Molica S, Mirabelli R, Molica M, Levato L, Mauro FR, et al. Clinical relevance and treatment of nonautoimmune anemia in chronic lymphocytic leukemia. Cancer Manag Res. 2011; 3: 211-217. Ref.: https://goo.gl/dwhQRz
- Smith MA, Shah NR, Lobel JS, Cera PJ, Gary GW, et al. Severe anemia caused by human parvovirus in a leukemia patient on maintenance chemotherapy. Clin Pediatr (Phila). 1988; 27: 383-386. Ref.: https://goo.gl/rPwSjU
- Visovsky C. Muscle strength, body composition, and physical activity in women receiving chemotherapy for breast cancer. Integr Cancer Ther. 2006; 5: 183-191. Ref.: https://goo.gl/7U4WiZ
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2014; 112: 438-445. Ref.: https://goo.gl/xj68Sd
- Bouaziz W, Vogel T, Schmitt E, Kaltenbach G, Geny B, Lang PO. Health benefits of aerobic training programs in adults aged 70 or over: A systematic review. Presse Med. 2017 46:794-807 Ref.: https://goo.gl/PvqE8a
- Willis WT, Brooks GA, Henderson SA, Dallman PR. Effects of iron deficiency and training on mitochondrial enzymes in skeletal muscle. J Appl Physiol(1985). 1987; 62: 2442-2446. Ref.: https://goo.gl/zLHkti